INTRODUCTION
Tumors of the brain are a serious problem in modern oncology. Gliomas and meningiomas occupy the leading places in the epidemiological structure of the primary tumors of the central nervous system (CNS) (35.5%), and 15.6% of them are glioblastomas (GBs) [1]. The morbidity rate with glial tumors depends on a histological type, age of diagnosis, sex, ethnicity, and geography. On average, the morbidity rate with all the types of glial tumors is 4.67–5.13 per 100,000 people. The morbidity rate with GBs averaged by the age is 0.89–3.69 per 100,000 people [1].
The main ascents in the classification of tumors of the CNS (the WHO, 2016) were made on the molecular-biological factors because genetic, epigenetic, and molecular-biological properties of the tumor contain the answers to the main unsolved issues of diagnostics and treatment. From the diagnostic and therapeutic points of view, different molecular links of oncogenesis are studied, like growth factors and their receptors, apoptotic and anti-apoptotic proteins, systems of DNA reparation, transcriptional factors, genetic mutative changes, and epigenetic modifications. At the same time, it is often impossible to identify one mechanism unique to a certain type of tumor. The individuality of a tumor is determined by a complicated mixture of different mechanisms and factors; thus, it is necessary to find markers that indicate not only the presence of a mutation but also its functional consequences, i.e. a marker has to be associated with certain proteotemic, genetic, or epigenetic alterations and precisely characterize the functional condition of the main cellular processes that are influenced by the above-mentioned alterations.
The most suitable markers are such proteins as protein kinase of different classes and the associated molecular factors. They are the main effectors of the majority of intracellular processes and play a key role in the realization of the main functions of tumorous cells significant for carcinogenesis. There were some studies dedicated to the effect of different protein kinases on the development of tumors of the CNS. However, the most significant drawback of those studies was their fragmentary nature and that they were not systemic. This is true for atypical isoforms of protein kinase C. Their role in the pathogenesis of glial tumors of the brain remains understudied. At the same time, this is a promising area for the research that can significantly change the approach to the diagnostics and treatment of glial tumors.
Atypical isoforms of protein kinase C do not require either Ca2+ or diacylglycerol for their activation. This family includes such protein kinases as protein kinase Cζ, protein kinase Mζ (PK Mζ), protein kinase C iota (PK Ci), and protein kinase Cλ [2]. These enzymes have unusual properties, in particular, higher catalytic activity than in other representatives of this class [2]. Since atypical isoforms of protein kinase C do not require additional co-factors for its activation, they can act not only as secondary mediators in the realization of different functions but can also act independently in the regulation of these functions, including metabolic and proliferative ones.
At the same time, PK Mζ is brain-specific and, unlike the above-mentioned protein kinases, is a key effector in the brain cells that realizes different intracellular functions. Besides, PK Mζ has a self-maintaining property of the activity, which defines the possibility of its constitutive and autonomic functioning [3] and the involvement of the key molecular cascades in the tumor. It should be mentioned that in the physiological conditions, PK Mζ maintains plastic transformations in cells and their stable functioning in the conditions of the changing functional load [3]. In other words, based on the biochemical and physiological properties of this protein kinase, it can act not only as the key effector in the functioning of several important metabolic, signal, and proliferative cascades in tumor cells but also as one of the central regulators of these processes realizing them in a specific manner.
When it comes to brain tumors, there were no studies on the role of PK Mζ in the development of these neoplasms. However, it was revealed that PK Mζ was involved in the processes of the proliferation of neurons in neurogenesis and can take part in proliferative cascades in cells of astrocytic glia [4]. These studies showed perspectives in the evaluation of the role of these protein kinases in the development of tumors of the brain.
High activity of PK Ci was observed in the cells of adenocarcinoma of the esophagus simulating the process of the proliferation of tumor cells and suppressing the processes of apoptosis in them [5]. For gastric cancer therapy, there are some promising drugs that inhibit this type of protein kinases. Besides, the role of PK Ci was shown in the development of other tumors. In some studies, the role of PK Ci was revealed in the development of GB [6]. This resulted in the fact that in cases of this type of tumors, PK Ci became the most promising object of therapeutic impact. For example, the application of aurothiomalate that reduced the productivity of a cascade pathway of PK Ci allowed the researchers to decrease the invasiveness of the tumor and initiate its regression [7]. The application of a special inhibitor of this type of protein kinases ICA-1 leads to a significant decrease in the volume of a tumor [8]. At the same time, there were no studies on the involvement of PK Ci in the pathogenesis of different types of glial tumors.
Thus, the study was aimed to evaluate and perform a comparative analysis of the activity of the expression of PK Mζ and PK Ci in diffuse astrocytomas (DAs), IDH-mutant anaplastic astrocytomas (AAs), and GBs of IDH wild-type and to establish the association between the activity of the expression of these factors and the prognosis for a patient.
MATERIALS AND METHODS
General characteristic of the study
The samples of IDH-mutant DA, IDH-mutant AA, and IDH wild-type GB were obtained from patients during a surgical intervention at the Burdenko National Medical Research Center of Neurosurgery in 2014–2016. In the postoperative period, patients underwent regular follow-up examinations and received standard chemo and radiotherapy. At the time of surgery, the average age of patients with DA was 38.28±4.84 years old, with AA – 42.48±2.32 years old, with GB – 62.46±4.28 years old. The average recurrence-free survival (RFS) in patients with DA was 48.54±8.34 months, in AA – 36.28±4.52 months, in GB – 8.62±2.38 months. On average, overall survival (OS) in patients with DA was 145.52±18.84 months, with AA – 124.48±16.54 months, in GB – 18.26±4.46 months. Materials from all patients were thoroughly studied by pathohistological and molecular-genetic methods for the verification of the diagnosis. Totally, the study included 60 samples of DA, AA, and GB each.
The study protocol followed guidelines for experimental investigation with human subjects in accordance with the Declaration of Helsinki and was approved by the ethics committee. Written informed consent was obtained from each patient (or official representative) before the study.
Immunohistochemical study
Tumor samples fixed in paraffin blocks were used to cut 3 µm sections. They were dewaxed with xylene and repeatedly hydrated with different concentrations of ethanol. The sections were dried in a thermostat at 45 °C. Further, the sections were consecutively incubated with rabbit monoclonal antibodies against the gene of human PK Mζ (Abcam, Great Britain) and conjugated with mouse anti-rabbit IgG-HRP (Cell Marque, Sigma-Aldrich, USA). Similarly, the sections were consecutively incubated with rabbit monoclonal antibodies against the human antigen of PK Ci (Abcam, Great Britain) and conjugated with mouse anti-rabbit IgG-HRP (Cell Marque, Sigma-Aldrich, USA). Antibody-binding sites were visualized with 3,3’-Diaminobenzidine tetrahydrochloride hydrate (Ventana Medical Systems, USA), nuclei cells were hematoxylin-stained.
Processing of the results of the immunohistochemical study
The samples were studied under a light microscope at an x400 magnification by three experienced pathologists by a blinded method. The activity of the expression of PK Mζ and PK Ci was evaluated by a semi-quantitative method. The share of cells with weakly positive staining (low activity of the expression) was multiplied by one. The share of cells with moderately positive staining (average intensity of the expression) was multiplied by two. The share of cells with strongly positive staining (high intensity of expression) was multiplied by three. After that, the obtained results were summed by the method of histoscore. The data obtained by three pathologists were averaged.
RNA extraction
RNA was extracted totally from tumor samples fixed in paraffin blocks using the AllPrep DNA/RNA Kit (Qiagen, Hilden, Germany). The concentration and quality of the extracted RNA were measured with a spectrophotometer NanoDrop 1000, RNA samples were stored at -80 °C before the analysis. The integrity of RNA was evaluated using theAgilent Bioanalyzer (Agilent Technologies, USA).
Quantitative polymerase chain reaction
The expression of PK Mζ and PK Ci was analyzed in all 180 tumor samples using quantitative polymerase chain reaction (PCR)with reverse transcriptase (qRT-PCR). The primers were constructed and IDT was synthesized (USA). All tumor RNA (1 µm) was reversely transcribed using a cDNA reverse transcription kit (LifeTech, USA). qRT-PCR was conducted using SYBR Select Master Mix (LifeTech) in a 384-well plate and QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, USA). Each 10 µm of the PCR mix contained 0.5 µl of the cDNA matrix, 200 nm of primers, and 5 µl of the SYBR master mix for PCR. The reaction included the stage of heating to 50 °C for 2 minutes. Further, the samples were heated to 95 °C for 2 minutes for the activation of Taq-polymerase, 40 cycles at 95 °C for 15 s, 60 °C for 60 s, and 60 °C for 1 minute. For each sample, the threshold cycle (Ct) was estimated in three variants. The expression of PK Mζ and PK Ci was normalized to GAPDH. For the evaluation of the relative activity of the expression, the index of expression was used calculated by the formula:
1000×2(-ΔCt), (1)
where ΔCt = Ct (PK Mζ or PK Ci) – Ct (GAPDH) for PK Mζ and PK Ci, respectively. Primers for PCR had a nucleotide sequence 5′- TGAAGGTGACCCTTGTACTGTG-3′ (direct) and 5′-CGGTATAGCTTCCTCCATCTTC-3′ (reverse) for PK Mζ, 5′-GAAGGCCACATTAAACTCACTGACTAC-3′ (direct) and 5′-GGTTGTATCTCCTGGCCGTAAT-3′ (reverse) for PK Ci, and 5′• GTCAAGGCTGAGAACGGGAA• 3′ (direct) and 5′• AAATGAGCCCCAGCCTTCTC• 3′ (reverse) for GAPDH.
Statistical analysis
The comparison of two average samplings was performed using the Mann-Whitney U-test. The association between PK Mζ and PK Ci, and RFS and OS in different types of diffuse gliomas were analyzed using the Kaplan-Mayer method and Log-test. For the estimation of relative risk (RR) and 95% confidence interval (CI) between the risk of recurrence or death and the activity of the expression of PK Mζ and PK Ci, the authors used univariate and multivariable regression models for Cox regression. All statistical tests were performed using the software SPSS Statistics 22 (IBM, USA). The values were statistically significant at p<0.05.
RESULTS
Results of the immunohistochemical study of the expression of PK Mζ
The present study was aimed to evaluate the activity of the expression of PK Mζ in DA, AA, and GB with a semi-quantitative immunohistochemical method. It was revealed that the activity of the expression of PK Mζ in DA was 46.48±2.34%. In AA, this parameter was 68.23±4.38%; in GB, it was 110.46±4.37%. At the same time, the activity of the expression of PK Mζ was significantly lower in DA in comparison with AA (p=0.00358; Z=4.32) and GB (p<0.00001; Z=5.12). Besides, the activity of the expression of PK Mζ in AA was significantly lower than in GB (p=0.00004; Z=4.86).
Thus, the highest activity of the expression was observed in GB. Further, AA was in the rank by the level of the activity. In DA, the expression was the lowest (Fig. 1).
Results of the immunohistochemical study of the expression of PK Ci
Besides, the authors studied the activity of the expression of PK Ci in DA, AA, and GB. It was shown that the activity of the expression of PK Ci was 45.46±2.38% in DA; in AA, this parameter was 48.26±4.53%, and in GB, it was 108.44±4.71%. At the same time, the activity of the expression of PK Ci did not differ significantly in DA in comparison with AA (p=0.08674; Z=2.38) and differed significantly from GB (p<0.00001; Z=5.14). Besides, the parameters of the expression of PK Mζ in AA was significantly lower in comparison with GB (p<0.00001; Z=5.16).
In general, the lowest values of the activity of the expression of PK Ci were observed in DA and AA, while in GB, this parameter was significantly higher (Figure 1).
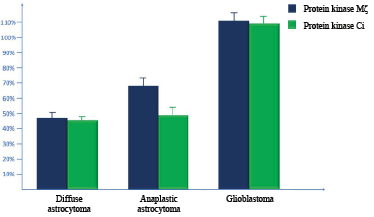
Fig. 1. Evaluation of the activity of the expression of protein kinase Mζ and protein kinase C iota in diffuse astrocytomas, anaplastic astrocytomas, and glioblastomas by a semiquantitative method (histoscore)
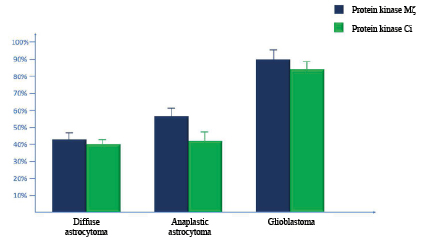
Fig. 2. Results of quantitative real-time PCR for mRNA protein kinase Mζ and protein kinase C iota in diffuse astrocytomas, anaplastic astrocytomas, and glioblastomas
Results of quantitative real-time PCR for mRNA PK Mζ
Real-time PCR (RT-PCR) revealed that the content of mRNA of PK Mζ was significantly higher in GB in comparison with DA (p=0.00034; Z=4.32) and AA (p=0.00186; Z=3.58). Moreover, this parameter in AA was significantly higher than in DA (p=0.00438; Z=4.48).
Thus, the highest expression of mRNA of PK Mζ was observed in GB; the lowest – in AA and DA (Fig. 2).
Results of quantitative real-time PCR for mRNA PK Ci
The authors performed quantitative RT-PCR to evaluate the activity of the expression of mRNA PK Ci in the studied tumor samples. It was shown that mRNA PK Ci was actively expressed in GB. The activity of the expression of mRNA PK Ci in GB was significantly higher than in AA (p=0.00052; Z=4.28) and DA (p=0.00048; Z=4.36). Still, there were no significant differences revealed by this parameter between AA and DA (p=0.08526; Z=2.14).
In general, the highest level of mRNA PK Ci was observed in GB. In AA and DA, this parameter was significantly lower (Figure 2).
Analysis of the influence of the activity of the expression of PK Mζ and PK Ci on the overall survival of patients
The analysis of the influence of the expression of PK Mζ and PK Ci on the prognosis of patients showed that the levels of mRNA and immunohistochemical activity of the expression of PK Mζ significantly influenced theOS of patients with DA (for immunohistochemical study p=0.00024, RR 0.29, 95% CI 0.09–0.90, for mRNA p=0.00032, RR 0.38, 95% CI 0.1-–0.8); AA (for immunohistochemical study p=0.00048, RR 1.0005, 95% CI 1.002–1.008, for mRNA p=0.00052, RR 1.03, 95% CI 1.025–1.035); and GB (for immunohistochemical study p=0.00564, RR 1.24, 95% CI 1.05–1.47, for mRNA p=0.00582, RR 1.20, 95% CI 1.01–1.43).
At the same time, both parameters of the activity of PK Mζ influence RFS only in patients with DA (for immunohistochemical study p=0.00438, RR 0.45, 95% CI 0.37-0.54, for mRNA p=0.00621, RR 0.63, 95% CI 0.55–0.71) and AA (for immunohistochemical study p=0.00071, RR 0.32, 95% CI 0.12–0.86, for mRNA p=0.00078, RR 0.42, 95% CI 0.08–0.94).
PK Ci was characterized by another consistency: the levels of immunohistochemical expression and mRNA significantly influenced only the OS of patients with GB (for immunohistochemical study p=0.00064, RR 0.56, 95% CI 0.16–0.88, for mRNA p=0.00056, RR 0.48, 95% CI 0.14–0.86). At the same time, the activity of PK Ci was associated with RFS also in patients with GB (for immunohistochemical study p=0.00448, RR 0.62, 95% CI 0.18–0.84, for mRNA p=0.00436, RR 0.36, 95% CI 0.08–0.92).
The authors were the first to perform a comparative analytical study of the activity of the expression of PK Mζ and PK Ci in diffuse gliomas of different degrees of malignancy. At the same time, it was shown that the activity of the expression of these factors gradually increased along with the degree of malignancy at proteomic and genetic levels. At the same time, this consistency demonstrated one exception for PK Ci: there was no significant difference between its expression in DA and AA. However, in general, this tendency was evident and could indicate a significant role of atypical isoforms of protein kinase C in the pathogenesis of gliomas. Besides, the obtained data agreed with the results of earlier studies conducted by the authors [9; 10].
The evaluation of the influence of the studied parameters on RFS and OS also revealed some interesting aspects. In particular, the activity of the expression of PK Mζ influenced OS in patients with all the studied tumors (DA, AA, and GB). At the same time, PK Ci significantly influenced OS and RFS only in patients with GB, which can indicate a more significant role of PK Mζ in the development and maintenance of malignant gliomas. At the same time, these data agreed with the published studies that showed the effectiveness of inhibitors of PK Ci in patients with GB [7; 8]. This fact can be taken as a clinical confirmation of the conclusions made by the results of the study. Still, further studies are needed in this area for the evaluation of the role of this protein kinase in the processes of carcinogenesis.
Besides, at the fundamental level, an interesting consistency was revealed. The present and previous studies showed that PK Mζ and PK Ci were significant for the pathogenesis of the majority of glial neoplasms. Such results can be explained by the origin of the majority of gliomas from neuronal stem cells. Earlier, it was proved that atypical isoforms of protein kinase C can be directly involved in the processes of neurogenesis and differentiation of neurons and glial cells at the stage of earlier precessors. Modern pathogenetic concepts on the mechanisms of the development of gliomas believe that such neuronal and glial precessors are a possible substrate for the formation of neoplasms. Thus, the obtained results can be associated not only with mechanisms of the pathology progression but also with the peculiarities of its appearance and development at earlier stages.
CONCLUSIONS
In the present study, the authors continued the study of the role of PK Mζ and PK Ci in the development and progression of gliomas. A comparative molecular study showed the perspectives for further fundamental studies and potential practical application. Fundamental studies should be dedicated to the mechanisms of the involvement of PK Mζ and PK Ci in the maintenance and growth of the biological aggressiveness of diffuse gliomas. In the clinical aspect, additional studies are needed for the development of effective diagnostic approaches based on the evaluation of the activity of the expression of PK Mζ and PK Ci and pharmaceutical drugs that inhibit their activity in glial cells. Further research will allow the authors to propose innovative and effective means of precise and target diagnostics in the therapy of gliomas in personalized medicine.
FINANCIAL SUPPORT AND SPONSORSHIP
The study was financed by the Russian Foundation for Basic Research within a framework of the scientific project No. 18-29-01034 mk.
CONFLICTS OF INTEREST The authors declare no conflict of interest



