INTRODUCTION
Breast cancer (BC) is the most pressing oncological issue for female patients. Various treatments have been proposed. Intraoperative radiation therapy (IORT) combined with surgery is an increasingly popular method that preserves the organ and allows the surgeon to reconstruct the breast. IORT is a term that refers to multiple different methods that vary substantially in terms of dose delivery and tissue exposure. Speaking of BC, 10-Gy (single exposure) intraoperative electron radiotherapy has been proven most effective as a method preceding whole-breast irradiation.
IORT minimizes the exposure and is gentle on the skin, which positively affects later tissue tolerance and therefore the appearance. This also reduces the occurrence rates of seromas and hematomas. The so-called boost IORT makes the surgery slightly longer; however, it significantly shortens postoperative radiation therapy. Breast preservation surgery coupled with postoperative whole-breast radiation therapy (WBRT) is today’s golden standard of early BC treatment. Several randomized studies have shown that postoperative radiotherapy has a substantial advantage in terms of the outcome due to lowering BC recurrence and mortality rates [2]. The Salzburg group did not have any recurrences at all; they showed an intraoperative electron boost to have a substantial advantage over the percutaneous boost coupled with WBRT. Five-year follow-up had a 0% ipsilateral breast tumor recurrence rate compared to 4.3% in the percutaneous irradiation group [3].
In some patients, the tumor bed itself is at higher risk of recurrence, which is why an additional increasing dose of 10 to 16 Gy (5 to 8 × 2 Gy) does reduce the local recurrence rates, making it recommendable for younger patients, as well as for patients at risk for other factors associated with higher local recurrence risks [4, 5]. Boost is implemented in a variety of methods including external radiation therapy, kV-IORT, and electron IORT [6].
There are several different immunophenotypic variations in BC. Four variations are singled out: two luminal variations (A and B), HER2+ and triple-negative (TN) molecular subtypes of BC. The luminal types express estrogen receptors and progesterone receptors; depending on Her2/neu expression, they are classified into Type A (no expression) and Type B (Her2/neu expression). These are also referred to as estrogen-dependent tumors, and they are the most common BCs (30% to 45% of all cases). Tumors begin to grow in the inner, luminal cells that line the milk ducts.
There are tumors that have none of the three features above; they constitute the so-called triple-negative or basal-like BC. Many researchers have shown that the luminal types have a less aggressive course and a relatively favorable prognosis compared to HER2+ and TN. Triple-negative BC (TNBC) is associated with a high BRCA1 mutation rate, has an aggressive course, does not respond to hormone therapy or trastuzumab; both the general survival rate and recurrence-free survival rate are low [7].
Today’s research focuses on how immunohistochemical markers correlate with the tumor sensitivity to chemotherapy and targeted therapy, as this helps individualize the treatment.
The goal hereof was to assess the post-IORT efficacy and toxicity where IORT was used as a boost for early BC patients.
MATERIALS AND METHODS
Early BC patients were followed up by the Almaty Cancer Center over three years from February 1, 2015 to December 31, 2017. For IORT, the Center used an Elliot LIAC unit made by S.I.T. (Sordina IORT Technologies S.p.A.), Italy. This unit can be integrated in any operating room without rearranging it; it does not require special radiation protections nor any special structures in the room. The unit weighs only 400 kg. Nominal electron-beam energy is 12 MeV; the unit is able to provide radiation therapy of virtually any complexity and to deliver 6, 8, 10, or 12 MeV of energy. The exposure it provides only requires 40 to 120 seconds of irradiation. The dose varies from 0 to 30 Gy/min.
The following inclusion criteria were applied: (1) 40 years of age or younger; (2) small tumor size (T) of 2.5 cm or less, no multicentric growth; (3) regional lymph nodes negative or one affected lymph node (N0 or N1); (4) positive receptor status identified by immunohistochemistry. The main group comprised 78 BC patients, Stages I to IIa. The patients were aged 38 to 72 or 58.8±11.8 on average. Prior to therapy, all early BC patients had had a standard set of tests: general clinical blood and urine tests, biochemical blood and urine tests, and functional tests of various organs and systems including instrumental tests.
To test the tumor histologically and confirm it morphologically, all 78 patients had trephine biopsy. Once histological test results were available, patients would undergo a mandatory histochemical test to detect the phenotypic variation of BC. Further treatment was subject to consultation involving an oncologist, a radiologist, a surgeon, and a chemotherapy specialist.
The study protocol followed guidelines for experimental investigation with human subjects in accordance with the Declaration of Helsinki and was approved by the ethics committee. Written informed consent was obtained from each patient (or official representative) before the study.
The study was of the retrospective design. After the surgical excision of the tumor (quadrantectomy and sectoral resection), the operating field was exposed to a single dose of 10 or 21 Gy without leaving the room. The local irradiation dose depended on the tumor phenotype. Thus, Luminal A patients (n=40) had 21 Gy without subsequent whole-breast irradiation. Luminal B (n=20), TNBC (n=12) and Her2neu-positive (n=6) patients had a single dose of 12 Gy. These patients further received WBRT at 30 to 35 Gy on Day 10 to 12 after surgery.
Patient reports including diagnostic tests and toxicity studies were analyzed after surgery and six to eight weeks after IORT or WBRT. The cosmetic effect was assessed on a 4-step scale by the Joint Center for Radiation Therapy [8]. General and event-free survival rates were calculated using the Kaplan-Meier method.
RESULTS
Table 1 shows the clinical characteristics of the early BC patients and their tumors. The left breast was nearly as likely to be affected as the right breast: 46.2% vs 53.8%, respectively. Stage T0 was not detected in any patient prior to surgery; however, final histological tests showed carcinoma in situ in three patients. T1a was identified in 10.2% of preoperative patients and 15.4% of postoperative patients. This indicated a slight shift in the stage. T1b and T1c stages showed little to no change. However, T2 was characterized by reverse shifts. Whilst 22 patients (28%) were diagnosed with Stage II BC, only 15 (19.3%) had this diagnosis after surgery, a sign of overdiagnosis. The same pattern was observed for T0 lymph node status: 72 BC patients had T0 identified by clinical tests preoperatively, but only 64 did postoperatively. N1 was underdiagnosed though: 7.7% and 16.6%. Notably, N2 was never diagnosed in preoperative patients and was diagnosed once in a postoperative patient.
Therefore, 16 out of 78 patients or 20.5% had different test results for biopsy samples vs surgical samples of pathological tissue:
– a higher T status in 4 patients (5.1%), a lower T status in 8 (10.2%) patients. No change in the remaining 66 (84.6%) patients;
– a higher N status in 5 (6.4%) patients, 1 (1.3%) of whom received neoadjuvant chemotherapy. A lower N status in 6 (7.7%) patients, of whom 4 (5.1%) received preoperative chemotherapy. No change in stage in other patients.
For irradiation, special applicators were applied to the tumor bed site (the excised tumor section), see Fig. 1.
Table 1
Patient and tumor characteristics
|
Total (n=78) |
n (%) |
n (%) after surgery |
|
Average age, years (rank) |
58 (38–72) |
|
|
Side |
||
|
Right |
42 (53.8) |
|
|
Left |
36 (46.2) |
|
|
T |
cT |
pT |
|
T0 |
– |
3 (3.8) |
|
T1a |
8 (10.2) |
12 (15.4) |
|
T1b |
15 (19.2) |
16 (20.5) |
|
T1c |
33 (42.3) |
32 (41.0) |
|
T2 |
22 (28.0) |
15 (19.3) |
|
N |
cN |
pN |
|
N0 |
72 (92.3) |
64 (78.0) |
|
N1 |
6 (7.7) |
13 (16.6) |
|
N2 |
0 (0.0) |
1 (1.4) |
|
Grade |
Biopsy |
Histology |
|
G1 |
24 (30.8) |
21 (26.9) |
|
G2 |
40 (51.3) |
44 (56.4) |
|
G3 |
14 (17.9) |
10 (12.8) |
|
Not diagnosed |
– |
3 (3.8) |
|
Immunophenotype Luminal A Luminal B Triple-negative Her2+ |
40 (51.3) 20 (25.6) 12 (15.4) 6 (7.7) |
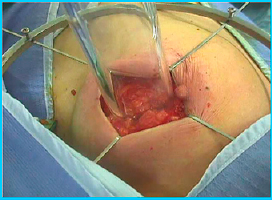
Fig. 1. Applicator being applied for intraoperative radiation therapy
Table 2
Treatment received
|
Total (n=78) |
n |
% |
|
Organ preservation surgery |
78 |
100.0 |
|
Axillary lymphadenectomy |
12 |
15.4 |
|
Neoadjuvant chemotherapy |
14 |
17.9 |
|
Adjuvant chemotherapy |
18 |
23.1 |
|
Adjuvant hormone therapy |
75 |
96.1 |
|
Remote radiation therapy of the affected breast |
38 |
46.8 |
|
Intraoperative radiation therapy |
78 |
100.0 |
Table 2 describes the BC patients in terms of received treatment. All patients received IORT and preservation surgery: breast resection coupled with a variety of plastic surgeries. TNBC patients had preoperative chemotherapy, mainly anthracycline-based (n=14). Some patients who had suspicious lymph nodes in the regional zones (the armpit) had the axillary lymph nodes dissected (n=12).
Thus, about half of the patients (46.8%) had remote radiation therapy of the affected breast. These were early BC patients that had Luminal B, TNBC, or Her2-positive cancer. WBRT was performed after surgery and IORT for a total of 30 to 35 Gy. Therapy used the standard fractionation method.
After IORT, some BC patients had adverse effects and complications typical of the procedure. Severe post-IORT fibrosis was not reported. However, two patients (2.6%) had mild or moderate fibrosis. Breast tissue necrosis described in literature was not observed in this study. Postoperative wound hematoma is a frequently noted adverse effect of IORT and surgery. This complication was also rare in the study: one case (1.3%). There was also a single case of skin scarring (1.0%). As for inflammation, two patients had wound infections that did not require surgery (2.6%) and were managed by anti-inflammatory (antibiotic) therapy. Thus, the complication rate was quite low and perfectly acceptable for this kind of intervention. The complication incidence rate totaled 7.6%.
The follow-up median was 33.6 months. Local cancer recurrence, progression, or continued tumor growth was not reported. The method was noted to have favorable cosmetic outcomes: no or insignificant breast asymmetry in 55 (70.5%) patients; some patients had satisfactory outcomes: pronounced asymmetry and 2nd degree radiation damage to the skin in 23 (29.5%) patients. No unsatisfactory outcomes (significant asymmetry, 3rd or 4th degree skin damage) were reported.
No deaths as of the end of the follow-up. The survival rate totaled 100.0%. The event-free survival rate was also 100% by the end of the study, where an event stands for a local recurrence or death.
CONCLUSIONS
The goal hereof was to assess the post-IORT efficacy and toxicity where IORT was used as a boost for early BC patients treated at the authors’ clinic. The study has shown IORT to be a safe method with mild to no side effects.
No severe toxicity was detected. However, literature data suggests IORT is associated with higher occurrence rates of seromas, infections, and wound complications compared to surgery alone. The seroma index seems consistent with the results of this study. However, most of the patients were not found to have wound infections or slower wound healing, which is why seroma formation rates were comparable to literature data. Some other authors also report no increase in seroma occurrence even at higher IORT doses. Thus, IORT is a safe and well-tolerated boost that is not likely to cause more perioperative complications. Moreover, the method reduces or even nullifies the rate of local recurrence, which is crucial from the standpoint of cancer treatment.
CONFLICTS OF INTEREST The authors declare no conflict of interest
SUPPLEMENTARY DATA (DOI)



