INTRODUCTION.
In recent years, ever more patients experience infections after hip replacements, which is due to (a) more surgeries being performed; (b) compromised immunity; (c) the emergence of numerous antibiotic-resistant pathogen strains [1–3]. Periprosthetic infections occur in up to 40% of all revision joint replacements [4, 5]. This is why the medical community today is focused on addressing these issues.
Most methods fail to adequately handle purulent complications emerging later after implanting, are not efficient, and yield positive results in 27–30% of all cases at best [6]. This is why an optimal treatment of chronic periprosthetic infection is believed to best involve an optimal antibacterial therapy and radical surgical treatment of the pus pockets, removal of the implant and bone cement, long drainage, and placement of an antibiotic-loaded spacer [7–9]. Antibiotic-loaded cement spacers are used in Stage I treatment of hip prosthesis infection immediately after the implant has been removed [10–12]. These devices provide the necessary concentration of antimicrobials in the inflammation zone while also filling the hollows of the acetabulum and proximal femur. Some authors report spacers to have an efficiency of 89% to 100% [13].
Li. et al. found spacers effective in 26 patients [14]. Berasi reports 28 surgeries using custom acetabular components. In all cases, the authors report good treatment outcomes and the absence of implant destabilization or displacement [15]. Precision 3D printing can produce custom, patient-tailored implants of any shape.
The goal hereof is to evaluate the outcomes of surgery using custom articulating hip spacers.
MATERIALS AND METHODS
The research team analyzed data of 67 patients who experienced periprosthetic infection after hip replacement and were treated at the Septic Surgery (Osteology) Unit of the Volga Federal Medical Research Center in 2015–2017. Patients underwent two-stage revision hip replacement, this time with spacers. The criterion for inclusion: the presence of an acetabular defect. Patients were split into two groups.
The control group comprised 33 patients: 23 men and 10 women. These patients’ treatment followed a standard two-stage revision hip replacement algorithm.
The treatment group had 34 patients (24 men and 10 women) that underwent revision hip replacement using customized spacers.
Patients were aged 62.4±12.2 years on average in the treatment group, 63.6±13.2 in the control group; there were no significant differences between the groups in terms of gender (p>0.05).
All patients had clinical and laboratory signs of post-replacement purulence, a fistulous wound with purulent drainage, and pathogenic microflora growth in arthrocentesis samples. Microbiological tests of such samples or wound discharge were run by the Laboratory of Bacteriology, Privolzhsky Research Medical University (PRMU).
Testing the etiological structure of the infection pathogens showed that in both groups, gram-positive microorganisms were more frequent than gram-negatives. Most of the identified gram-positive bacteria were staphylococci, where methicillin-resistant S. aureus (MRSA) strains accounted for 52.2% of the pathogens (54.5% in the control group and 50% in the treatment group); coagulase-negative staphylococci (MRSE) accounted for 28.4% (27.3, 29.4). The microbiological spectrum did not differ significantly in these groups (p>0.05).
X-ray tests were run by the X-Ray Unit of the PRMU using a Dira-RC machine in frontal and lateral views; plan radiography of the pelvis was made in the anteroposterior view. The physicians analyzed the location of implant components, the presence of bone defects, and the stability of the implant.
Bone defects of the acetabulum were found in all patients of both groups and were scored by the Paprosky scale, see Table 1.
Table 1
Bone defects of the acetabulum on the Paprosky scale
|
Indicator |
Control group (n=33) |
Treatment group (n=34) |
Total: |
|||
|
Abs. |
% |
Abs. |
% |
Abs. |
% |
|
|
I I B |
18 |
54.5 |
17 |
50 |
35 |
52.2 |
|
I I C |
9 |
27.3 |
10 |
29.4 |
19 |
28.4 |
|
I I I A |
5 |
15.2 |
6 |
17.7 |
11 |
16.4 |
|
I I I B |
1 |
3 |
1 |
2.9 |
2 |
3 |
Thus, the groups were similar in terms of acetabular bone defects (p>0.05).
Where there were signs of bone defect in the acetabulum, most patients of the control group (21 of 33) and all the patients of the treatment group underwent computed tomography. Knowing the size and anatomy of the defect helped design the future spacer while preparing for surgery.
In the control group, acetabular bone defects were corrected with different spacers: an articulating spacer, a Tecres officinal spacer, and the bipolar spacer, Patent No. 142311 of May 21, 2014 of the authors' own design, see Figure 1.

Fig. 1. Bipolar hip spacer
Note that the team’s in-house developed surgery tactics pursued excluding the bone from articulation at the first stage of repeated hip replacement. Cement-bone friction pairs are often associated with secondary bone tissue defects, which complicates the second stage of surgery. This is why it is always desirable to implant a truly articulating spacer that would not engage bone structures in articulation. Unfortunately, given the control group’s bone defects, this was sometimes unavoidable, see Table 2.
Table 2
Types of spacers in the control group
|
Spacer type |
Articulation in the cement-bone system |
Bone not involved in articulation |
|
Articulating |
0 |
17 |
|
Officinal |
5 |
0 |
|
Bipolar |
11 |
0 |
|
Total: |
16 |
17 |
Officinal and bipolar spacers are clinically inefficient, resulting in progressing bone defects and bone dislocations before the second stage of surgery. To address that, the research team behind this paper designed a new method for hip spacer implantation to correct acetabular defects, see Patent RU No. 2632525 of October 5, 2017. This device effectively excludes the bone from articulation and helps reduce the prolapse of spacer components in the lesser-pelvic cavity to lower the risk of damaging the anatomical structures.
The designed spacer was implanted as follows. Once X-ray test results were confirmed, patients underwent pelvic CT to collect data from which a special application could retrieve information that would help restore the image of bone structures, while the intensity gradient ratio was set empirically at 0 to 225, see Figure 2.
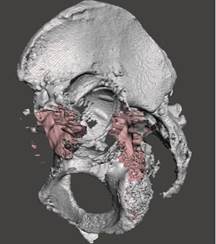
Fig. 2. Computer 3D model of the acetabulum
Then voxels were filtered that contained data on the patient’s implant, see Figure 3.
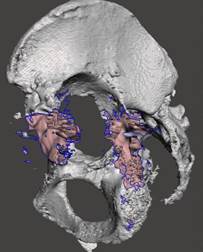
Fig. 3. Computer simulation. Acetabular component of the implant removed in the rendering
Based on this data, the research team ran hybrid parametric modeling of the damaged hip. In the case of a bone defect, it was reconstructed using, among other things, a mirror copy of the contralateral acetabulum. A hemisphere of 0.5 to 1 cm in wall thickness was placed at the true center of rotation to place a standard cement-implant cup on bone cement.
To further obtain the volumetric parameters of the modeled acetabular spacer component, the team generated computer models of the mold that would be sent over a remote connection or copied via a hard medium to an FDM 3D printer to make that mold of the Hips material. 3D printing was provided by FDM 3D printers manufactured by Makerbot replicator 2x, USA, and Ultimaker 2+, the Netherlands.
The newly made mold was sterilized, filled with antibiotic-enriched bone cement intraoperatively, and then dismantled once the cement cured, see Figure 4.
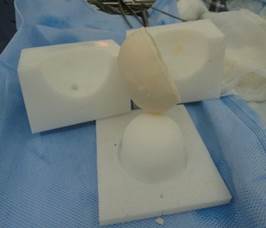
Fig. 4. Intraoperatively created acetabular spacer component and the mold for making it
Custom-made acetabular spacers were implanted in the existing defect onto bone cement with an antibiotic, see Figure 5.
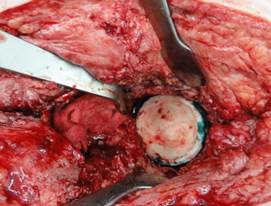
Fig. 5. Implanted custom acetabular spacer
A polyethylene acetabular component of the implant was impacted in the placed custom spacer in an appropriate position, and then secured with antibiotic-enriched bone cement, see Figure 6.
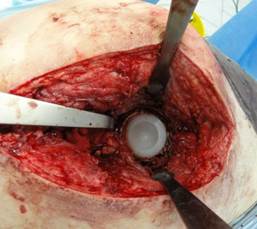
Fig. 6. Implanted polyethylene acetabular component of the implant
Further surgery was standard and included placing the femoral component of the implant on the bone cement, adjusting it, doing X-ray scans, and stitching the wound.
Thus, using custom acetabular spacer components attained the main goal of surgery, which was to exclude the bone from articulation in most cases, see Table 3.
Table 3
Types of spacers in the treatment group
|
Spacer type |
Articulation in the cement-bone system |
Bone not involved in articulation |
|
Articulating |
0 |
29 |
|
Officinal |
3 |
0 |
|
Bipolar |
2 |
0 |
|
Total: |
5 |
29 |
Unfortunately, articulating and officinal spacers had to be used in some cases. However, the authors believe there were fewer indications to use them. Today, the authors’ clinic only uses long-legged officinal spacers if there is an accompanying pronounced defect of the proximal femur, Paprosky Type 3, 4, see Figure 7.
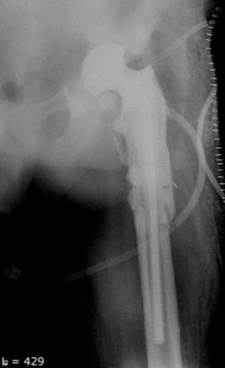
Fig. 7. Implanted officinal hip spacer, proximal femur defect, Paprosky Type 4
Bipolar hip spacers were only used in the treatment group in the case of pelvic disjunction or signs of infection in the lesser-pelvic cavity, see Figure 8.
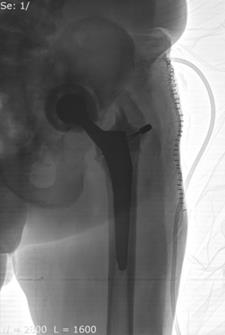
Fig. 8. Implanted bipolar hip spacer: pelvic disjunction
After surgery, both patient groups underwent antibiotic therapy based on their antibiograms, as well as anticoagulant therapy to prevent thromboembolic complications.
Based on the estimated functional status of periarticular tissues, the type of spacer used, and the signs of inflammation (present or not), patients were "activated" on Day 5 to 7 after surgery. The drainage was removed until verticalization. Patients were guided to walk on crutches partially loading the operated limb. Isometric gymnastics was mandatory to preserve periarticular muscle balance.
The follow-up consisted of local status checks, laboratory test assessments, and taking arthrocentesis samples for bacteriological tests. In the case of no signs of inflammatory manifestation, the patient would be directed to Stage II revision hip replacement.
Once the spacer was in place, the patients would be assessed by the Harris hip score in a month and then in three months after spacer placement; they were also asked if they had been taking painkillers, what kind of labor they did, and whether they used walking supports.
STATISTICA for Windows 8.0 was used for statistical processing of the obtained data.
The study protocol followed guidelines for experimental investigation with human subjects in accordance with the Declaration of Helsinki and was approved by the ethics committee. Written informed consent was obtained from each patient (or an official representative) before the study.
RESULTS
It was found out that 2 patients of the treatment group (6.7%) and 4 controls (13.3%) needed repeated post-revision surgery and spacer replacement, with no statistically significant difference (p>0.05). Repeated suppuration of the spacer location as found by bacteriological testing was considered an indication for spacer replacement. Tests were run at 30, 60, and 90 days after surgery.
Harris hip scoring found no statistically significant original difference between the groups, see Table 4. One month later, the treatment group had a somewhat higher score for pain, function, and range of motion; the difference was insignificant. It was only three months after surgery that the total Harris score was significantly higher (p<0.05) in the treatment group than in the controls.
Table 4
Preoperative Harris hip scores
|
Score item |
Control group (n=33) |
Treatment group (n=34) |
|
Before surgery |
||
|
Pain (max. 44 points) |
23.1±3.2 |
25.2±4.7 |
|
Function (max. 47 points) |
26.8±4.6 |
28.4±3.0 |
|
Absence of deformity (max. 5 points) |
1.4±0.4 |
1.8±0.5 |
|
Range of motion (max. 4 points) |
1.1±0.2 |
1.2±0.3 |
|
Total (max. 100 points) |
52.4±3.9 |
56.6±5.1 |
|
1 month after surgery |
||
|
Pain (max. 44 points) |
35.2±3.8 |
38.8±2.6 |
|
Function (max. 47 points) |
36.4±3.3 |
41.5±3.0 |
|
Absence of deformity (max. 5 points) |
2.1±0.9 |
3.1±1.2 |
|
Range of motion (max. 4 points) |
1.8±0.3 |
2.1±0.2 |
|
Total (max. 100 points) |
80.4±3.2 |
86.5±2.8 |
|
3 months after surgery |
||
|
Pain (max. 44 points) |
39.1±4.1 |
29.4±4.2 |
|
Function (max. 47 points) |
41.4±3.7 |
45.7±3.1 |
|
Absence of deformity (max. 5 points) |
2.6±0.6 |
3.2±0.5 |
|
Range of motion (max. 4 points) |
2.4±0.4 |
2.8±0.2 |
|
Total (max. 100 points) |
85.5±3.9 |
96.3±3.6* |
Note: * stands for a significant (p<0.05) Group 2 vs Group 1 difference
Note that 39.4% of the control group and 29.4% of the treatment group were taking painkillers; 42% and 50% (p>0.034) had impaired motility.
DISCUSSION
The medical and social significance of hip pathologies has risen dramatically to date, as many countries currently have ever more elderly and senile patients [3, 5, 13]. Hip replacement is a popular surgery today; it is an efficient method that helps reduce the functional deficiency of limbs in such patients [9]. However, it often fails to perform as expected and is associated with infectious complications developing shortly after surgery, which requires a revision.
Given the ever-greater number of periprosthetic infections in patients, physicians diagnose more and more pronounced acetabular defects that require a nontrivial approach to surgery.
The developed method for implanting an articulating spacer using a custom acetabular component stabilized the implant and enabled bone-free articulation in 29 of 34 patients in the treatment group compared to 17/33 controls.
Full articulation and preservation of motility in the spacer site is the key to a functionally successful second stage of hip replacement as confirmed by the functional testing the patients underwent 3 months after Stage I. Their Harris scores were significantly higher when treated by the approach proposed herein.
Revisiting the frequency of repeated surgeries of the implant that required spacer replacement, the authors found that this was the case for 13.3% of the controls, 6.7% of the treatment group.
CONCLUSIONS
The data generally shows that the proposed treatment strategy that involves finding the exact scope of the bone defect and has an algorithm for the customization of an antibiotic-loaded spacer is an effective method that addresses the chronic purulent inflammatory process while improving the functional outcome.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
The authors declare no conflict of interest



