INTRODUCTION
Throughout the world, most oncological processes begin in the sixth or seventh decade of life, mainly affecting urban residents [1]. The incidence of melanoma in children and adolescents has been growing by an average of 2% per annum [2]. The melanoma incidence rates are higher in Australia than in similar European or Asian populations [3]. The increasing incidence of melanoma, which mostly occurs in the skin of elderly men, correlates well with the general clinical picture of melanoma and indicates that UV radiation is a major epidemiological factor. Hayward et al. (2017) state that skin melanomas are only common in Caucasians, whereas on a global scale, they generally tend to occur in the mucous membranes of internal organs, on hands or feet [4]. Malignant nodular melanoma is one of the most malignant tumors, and its aggressiveness is not bound to size. What causes melanoma and its progression is currently unknown; numerous concepts that exist are subject to much debate [5]. Nodular melanoma treatment is a complex surgery, and the prognosis is always unfavorable due to the risk of early metastasis. Complete surgical excision of melanoma together with some of the normal skin surrounding the tumor is recommended. Surrounding tissue within a radius of 5 mm must be excised, because melanoma tends to have regional metastases in lymph nodes or bypassing them. At virtually any stage, treatment does not increase survival regardless of the degree of tumor invasion. It is therefore important to know how much of the surrounding tissue is to be removed. Malignization of skin melanoma has been studied to a greater extent on experimental animal models; however, mouse model data do not extrapolate well to humans [6]. The reason is that mice have melanocytes in their hair follicles. Another limitation of mice research lies with the changes in their immunity, as well as with their skin damage mechanisms, which causes an unnatural phenotype of melanoma progression. The only way to find out how much tissue around melanoma must be removed is to test the morphology of the tumor and the core microenvironments around it such as epidermis, dermis, basal membrane, blood vessels, and connective tissue. This is where human material becomes relevant [7]. Despite progress in diagnosing skin melanoma, whether proliferation is an unconditional morphological criterion of tissue malignization remains an open question. The available literature has no data on skin plasticity as affected by the melanoma pathogenesis; as to date, researchers still have not found out why the post-extirpation wound does not heal. Thorough research into the morphology of the tumor and its environment will complete the understanding of melanoma pathogenesis to identify and improve the scope of diagnosing and predicting melanocytic tumors [8].
The goal hereof is to find the morphological features of skin structure in the melanoma-affected region.
MATERIALS AND METHODS
The study involved 27 patients of an oncology clinic in Vladivostok from 2009 to 2017 aged 45 to 70; the research was carried out in full compliance with the Russian Ministry of Health and Medical Industry (Doc. No. 82 of April 29, 1994) and in accordance with the nomenclature of clinical laboratory tests of the Ministry of Health (Order No. 64 of February 21, 2000) following the Helsinki Declaration of 2013. The research team also used 38 cadaverous biopsy samples taken from the skin of deceased melanoma patients. Cluster analysis was applied to test the potential homogeneous subgroups of dermal melanomas following the principles of evidence-based medicine. The team applied classic morphological testing and stained the cuts with hematoxylin and eosin to further analyze the obtained indicative materials. For retrospective lesion assessment in terms of morphological features, the researchers used an Olympus Bx 52 microscope. The study was permitted by the Ethics Committee of the Pacific State Medical University and Far-Eastern Federal University.
RESULTS
Melanoma morphology was tested in tissue materials as functioning in its real environment. Macroscopically, the patients had vertical tumor growth with a gray tumor surface, ulcerations in the central zone, and infiltration around the malignization area. Histopathology also identified signs of malignant melanoma (Clark V, Breslow 45 mm). Microscopy identified pockets of destruction in all layers of skin with disrupted intercellular contacts and no basal membrane of the epidermis. Basal epitheliocytes were elongated in parallel to the skin surface. The basal membrane could not be identified. Tumor cells were adjacent to the basal layer; the papillary layer was occupied by the infiltrating tumor. There were no "loop-like" capillaries typical of the papillary layer. The tumor tissue was vascularized. The stratum spinosum was thinned, composed of only four cellular layers. The epidermis lacked tissue polarity, its surface showing pockets of necrosis, no stratum corneum, no stratum granulosum.
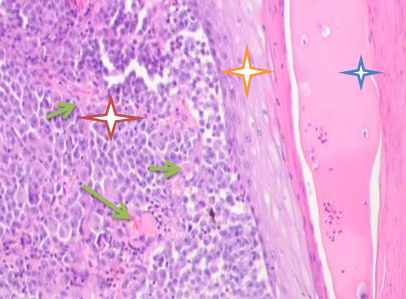
Fig. 1. Skin of a woman, 58. Nodular melanoma. Hematoxylin and eosin staining.
Microphotograph. Magnification: (a) x100; (b) x400. Epidermal destruction. No skin pattern on the melanoma surface
Notes: the red asterisk is for skin tumor; the orange asterisk is for thinned epidermis; the blue asterisk is for pathology-affected epidermal surface; the green arrows show multiple vessels in the tumor stroma.
The infiltration area had lymphocytes with basophil nuclei and a narrow cytoplasmic rim. Large cells that had oxyphilic cytoplasm without pigment inclusions or cytoplasm filled with melanin granules were located in stroma- and vessel-free segments, see Figure 2.
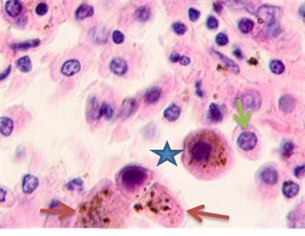 a
a 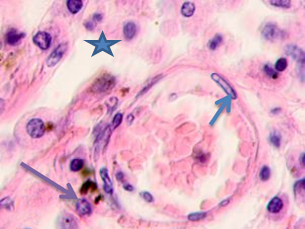 b
b
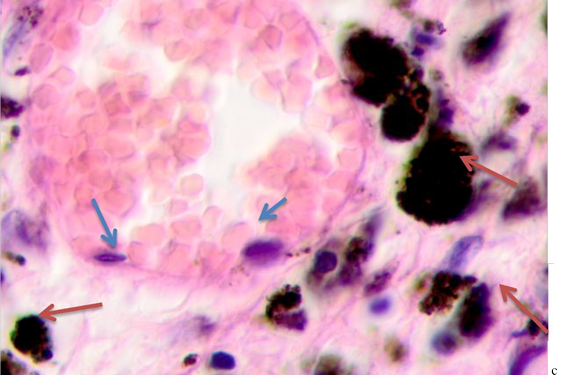
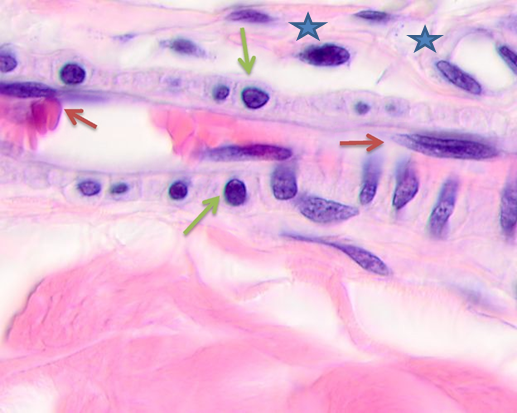
Fig. 2. Skin of a woman, 58. Nodular melanoma: (a) tissue within 5 mm around the tumor; (b) tissue at 7 mm around the tumor; (c) tissue at >7 mm around the tumor. Hematoxylin and eosin staining. Microphotograph. x100 magnification
Note: the blue asterisks show the infiltrates with plasmacytes indicated by green arrows; the red arrows point at melanocytes, and the purple ones point at apoptosizing; the blue arrow points at the endotheliocytes. No intercellular substance, the cells were in the exudate.
Detailed analysis into the epidermis at tumor boundaries showed changes in basal layers, apoptosis of the cambial cells, and destruction of the basal membrane. The stratum spinosum had an abnormal structure, the cells were rounded, and the boundaries between the stratum spinosum cells were not identifiable. Unlike in data of other researchers, the basophilia of the cambial layer nuclei was associated with karyopyknosis and apoptosis rather than proliferative activity. Cells in cambial layers formed multiple layers with lymphocytes identifiable in-between, see Figure 3.
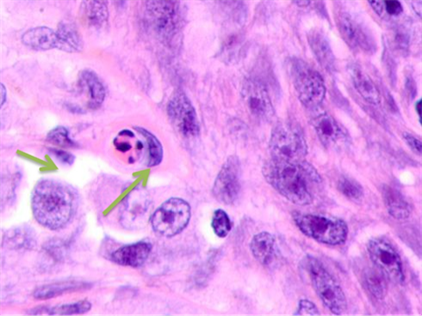 a
a
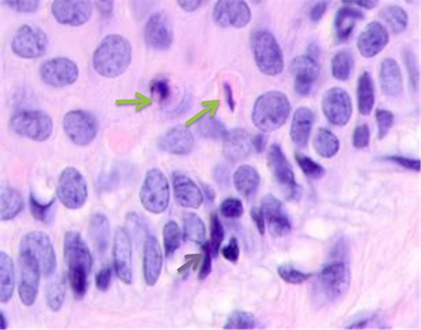 b
b
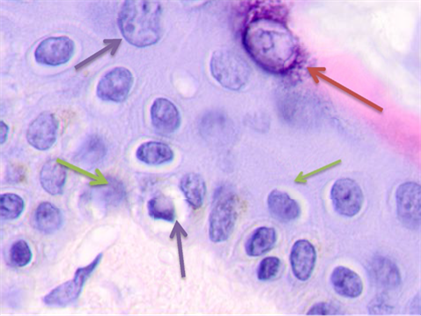 c
c
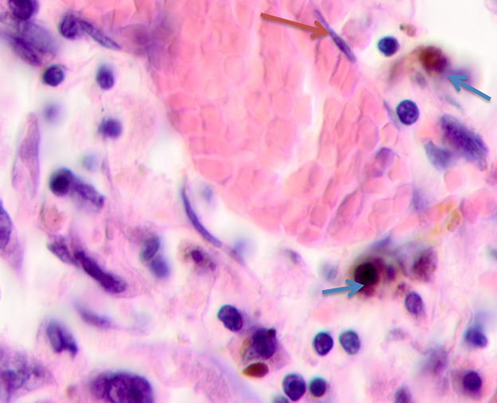
Fig. 3. Skin of a woman, 58. Nodular melanoma: (a) tissue at the tumor boundaries; (b) tissue within 5 mm of the tumor; (c) tissue at >7 mm off the tumor. Hematoxylin and eosin staining. Microphotograph. x100 magnification
Note: the green arrows point at the apoptosizing cells of the stratum spinosum, the purple arrow points at an apoptosizing basal keratinocyte, and the red one points at a stratum granulosum cell.
Melanin-containing epithelioid and fusiform tumor cells were found to be in active mitosis (3 to 5 mitoses in sight). The skin epidermis at >5 cm off the tumor had rare granular keratinocytes, 2 to 3 layers of spinous cells, sometimes clear-bordered. Up to 14 cells in sight were apoptosizing (50% of the total number, see Figure 2c); 2 to 3 cells contained melanin in their cytoplasm. Such data suggests that farther from the epicenter of malignization, keratinocytes of cambial layers undergo apoptosis, the epidermis is thinned, and its barrier functions degrade. Lack of the basal epidermis membrane in the epidermis and stratum granulosum, an atypically structured stratum spinosum, and apoptosis are all signs of cells lacking differentiation and maturation. The morphological analysis of tumor vessels and stroma showed that the vessels emerging in the tumor parenchyma did not have typical vascular walls. Erythrocytes and cells with large, low-basophilic nuclei were found in the vascular lumen. Lymphocytic infiltration was identified around capillaries. Endothelium was hypertrophied, partially destroyed. Some endothelium nuclei were fragmented, sharply basophilic with signs of apoptosis, see Figure 4. The stroma around the vessel and the cells was homogeneous.
The research team noted a high nuclear-cytoplasmic (NC) ratio.
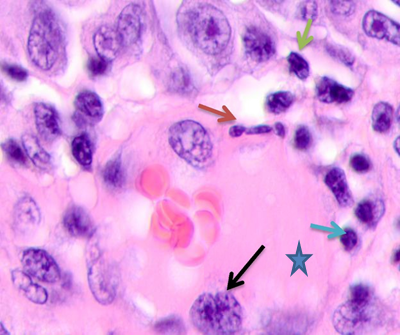
Note: the blue asterisk shows the vascular lumen; the red arrow points at an apoptosizing endotheliocyte; the blue arrow points at lymphocytes; the green arrow points at an apoptosizing cell; the black arrow points at a hypertrophied endotheliocyte.
Inappropriate differentiation and the apoptosizing cambium of keratinocytes and endothelium of the original tissue resulted in the migration of the foreign cells; the malignant tumor did not emerge from the proliferating cambium itself. Besides, the cells migrating to the tissue restructuring zone were not able to differentiate, as the apoptosizing keratinocytes cannot produce differentiation factors for progenitor cells. At >7 mm off the tumor, unlike in closer segments, the blood vessels of the papillary layer had intact vascular cells and flat endothelium with the vascular lumen being filled with erythrocytes and some rare blood cells, see Figure 5.
 a
a
 b
b
 c
c
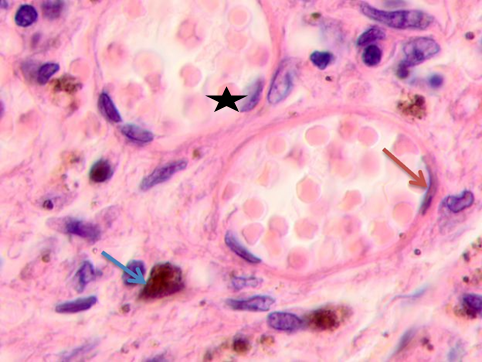
Fig. 5. Skin of a woman, 58. Nodular melanoma. Tissue at the tumor boundaries. Hematoxylin and eosin staining. Microphotograph. x100 magnification
Note: the blue asterisks show apoptosizing cells; the black ones point at hypertrophied endothelium; the red arrows point at flat endothelium, the green ones at cubic cells in the vascular wall, and the blue ones at melanocytes.
At >5 mm off the tumor, analysis identified vessels with typical flat endotheliocytes and a preserved basal membrane with adjacent cubic cells that had rounded basophilic nuclei, see Figure 5a. The microenvironment was mostly homogeneous intercellular substance and apoptosizing cells. Notably, the vascular walls remained intact at 5 to 7 mm off the tumor; they were lined with flat endothelium and had adjacent singular lymphocytes on the outside, see Figure 5b. Erythrocytes could be seen in the vascular lumen. The vascular microenvironment was mostly melanocytes, fibroblast-like cells, and lymphocytes. At >7 mm off the tumor, the papillary layer had capillaries with unaltered or slightly hypertrophied endotheliocytes, surrounded by melanocytes; up to 3 or 4 lymphoid migrants could be found in sight, see Figure 5c. Collagen fibers were found in the intercellular substance of the vascular lumen. Thus, farther from the tumor, the epidermal and dermal changes included keratinocytic apoptosis, destruction of the intercellular substance, lymphocytic infiltration, endotheliocytic hypertrophy and apoptosis, and greater melanocyte counts.
DISCUSSION
Kuzbicki et al. (2017) believe that the tumor thickness and the degree of infiltration of the surrounding skin, ulcers, a high mitotic index, a more invasive histological type, vertical growth phase, and lymph node metastases have a positive correlation with other prognostic factors that are cumbersome, difficult and costly to use [9; 10]. This information does not explain why on top of high proliferative activity, normal tumor excision at the boundary of the tumor and normal tissue creates a wound that does not heal for long. According to the authors’ data, this is due to a lack of skin restitution, which is attributable to apoptosis that propagates beyond the infiltration and the limits of the macroscopically intact skin. However, morphological analysis also showed that the malignization area was actually greater as indicated by apoptosizing cells. The risk of infection is due to lack of barrier function in the tissue surrounding the tumor, which in turn is attributable to the disrupted differentiation, while incomplete tumor excision leads to a greater risk of recurrence, as the extirpated tumor and its surrounding skin of the visible infiltration area do not match the actual affected area. Research has shown that a stronger anticancer immunity bolstered by regulating the suppressor immunity in cancer-affected areas is an important issue of improving the immunotherapy against cancer. Accordingly, recent years’ research in cancer immunology has shifted towards creating an immunosuppressive environment; studying the immunosuppression mechanisms not only in the tumor area but also in its surroundings; monitoring the cellular ensembles involved in the malignization process [11; 12]. Erdmann et al. (2017) point at tumor size, invasion depth, ulcers, age, and metastases in regional lymph nodes as the most significant signs for melanoma prognosis [13]. However, it should be borne in mind that a small tumor often produces satellite tumors early in the surrounding tissue and has early metastases in all internal organs while bypassing the regional lymph nodes. This is why the pathogenesis of some melanomas makes the Breslow and Clark criteria unsuitable, as they do not fully correspond to the actual morphological characteristics of melanoma. In this regard, integrating confocal microscopy and clinical/histological tests can help detect and treat a variety of tumors [14].
Melanoma is associated with not only malignant melanocytes but also altered relations between normal and neoplastic cells including fibrocytes, endothelial and inflammatory cells, and tumor stroma.
Infiltrated and surrounding fibrocytes, also known as cancer-associated fibroblasts (CAFs), differ from normal dermal fibrocytes in terms of phenotype and physiology. They become similar to myofibroblasts, reconstruct the extracellular matrix (ECM), and alter the structure of malignant tissue, secrete chemical factors and signaling molecules that boost the tumor growth, angiogenesis, inflammation, inflammation, and metastasis. Unlike Haridas et al. (2017) that found no clear evidence of any cellular interactions in the tumor beside contacts [15], the authors hereof believe that it is such interactions that trigger malignization. The loss of intercellular contacts and the inability to restitution are due to the processes of apoptosis of keratinocytes. Since the cells preserve their membranes, no signals pass that could induce restitution. The compromised barrier functions of the epithelium result in the inflammatory infiltrate invading the surrounding tissue; progenitor cells that are unable to differentiate due to lack of differentiation-inducing factors become prevalent. Morphological changes reflect the pathogenesis of the process in the tumor environment. Apparently, a combined therapy that targets the tumor and its surroundings could prospectively help overcome the resistance to treatment. What makes addressing these issues relevant is that the degree of surgical intervention for the removal of tumors and tumor-surrounding tissue remains debatable. The data presented herein shows that the scope of excising the tumor and its surroundings must be tailored to the patient by applying the principles of personalized medicines and the objective knowledge of the patient’s skin morphology and cellular composition of the tumor-adjacent tissues.
CONCLUSIONS
The general finding is that simple objective morphological scoring will suffice for making predictions with respect to skin melanoma patients; such scoring involves the histological testing of the primary melanoma area and its surroundings that contain inflammatory infiltrate at >10 mm off the tumor regardless of the tumor size. This research will help not only develop targeted immunotherapy against cancer but also predict the outcomes of melanoma by monitoring its microenvironment. Knowing the exact morphology of the boundaries separating the tumor and the healthy unaltered tissue will help the practitioners optimize the strategy and scope of surgery while also finding the accurate excision area. The authors’ data shows that it is not the lack of proper wound treatment that can trigger pathogenic microflora-induced inflammation, but the lack of barrier functions that the tissue around the tumor fails to perform.
Removing the tumor, the infiltration area, and the region of apoptosizing keratocytes will cover all the tissue that actually corresponds to the malignization area.
DISCUSSIONFINANCIAL SUPPORT AND SPONSORSHIP
Research was supported by the FEFU Research Foundation under the National Contract No. 17.5740/2017/6.7.
CONFLICTS OF INTEREST
The authors declare no conflict of interest



