INTRODUCTION.
Breast cancer (BC) remains one of the most significant medical-social problems. During the past decades, there was no decrease in the rate of primary diagnostics of this pathology. Worldwide, the morbidity rate is estimated at 46.3 per 100 000, which is more than 40% of all malignant tumors in women [1, 2]. There was no significant progress observed in the prevention of BC recurrence and tumor metastasis, which negatively influences the quality of life and survival of patients (the lethality rate is 13.0 per 100 000). It should be noted that about 19% of cases of BC are diagnosed in women aged 30 to 49 years, 44% of cases in women aged 65 years and older [2]. One of the achievements of the past decades was a decrease in the lethality rate among patients with BC in some developed countries, especially, in the young age, which is primarily associated with the implementation of programs of early screening of BC with mammography and the development of new and more effective anti-tumor drugs [3]. At the same time, in many developing countries, the lethality rate from BC is not decreasing or is growing, which provides a general increase in the lethality rate from BC during the past decades.
Such dynamics of breast cancer morbidity and mortality necessitates a comprehensive study of this pathology, especially risk factors, genetic predisposition, molecular mechanisms of tumor growth progression, tumor cell metastasis, as well as highlighting disease prognosis markers after surgical and/or chemotherapeutic treatment [4, 5]. Among the valuable prognostic markers of BC survival, along with the histological type of tumor and the degree of its malignancy, are the so-called involvement of regional lymph nodes in the tumor process, namely the presence or absence of metastases in them [6]. According to some authors, the involvement of 10 and more axillary lymph nodes in the tumor process increases the risk of lethality from BC within 10 years by 70% in comparison with those patients that have only 1-3 lymph nodes involved [7]. Thus, lately, the studies have been focused on the evaluation of the significance, reliability, and safety of the biopsy of axillary lymph nodes in patients with BC for the establishment of their metastatic status before and after neoadjuvant chemotherapy and the estimation of the effectiveness of treatment and possible life expectancy [8–10]. Not all authors highly value the diagnostic and prognostic significance of the biopsy of lymph nodes. It is often highlighted that it differs depending on the histologic variants of BC, the effectiveness of chemotherapy or the status of lymph nodes after chemotherapy [8, 11]. Still, all the researchers agree that further detailed studies of the lymph nodes in patients with BC are required to obtain additional information on the character of their structural reorganization, including the cases of non-metastatic tumors.
The analysis of available publications showed that the studies on regional lymph nodes are focused on the identification of their metastatic status and its change after chemotherapy. Other structural changes in the lymph nodes are not usually evaluated, which does not allow for the establishment of the whole spectrum of negative effects of the malignant tumor process, including BC, on the organs and tissues that are functionally and anatomically associated with neoplastic foci. A significant structural reorganization of lymph nodes, that are a part of peripheral organs of the immune system, can negatively influence the maturation of the immune-competent cells, which can aggravate the condition of oncological patients and decrease their quality of life after chemotherapy and surgical treatment [12]. An insignificant use of structural-functional characteristics of non-metastatic lymph nodes for the development of prognostic criteria of long-term survival and comorbid pathology taking into account the aggressiveness of the tumor process should be noted.
The study was aimed to evaluate the expression of the sclerotic process and the intensity of angiogenesis in the axillary lymph nodes in patients with different stages of breast cancer using the methods of morphometry and immunohistochemistry.
MATERIALS AND METHODS
The study included non-metastatic axillary lymph nodes that were dissected during mastectomy in 104 patients that underwent treatment at the department of breast care in 2009-2014 with the diagnosis “breast cancer”. According to TNM classification, there were patients with T1N0M0 (52 patients, aged 47.1±13.0 years old), T2N0M0 (43 patients, aged 47.5±12.9 years old) and T3N0M0 (9 patients, aged 50.0±11.3 years old). In all study groups formed in accordance with the stage of BC, patients aged 40–50 years accounted for about one third (respectively, 27, 28, and 33% of all patients in stages I, II, and III of BC). According to the histological structure, tumors were represented by non-infiltrating intraductal BC (14 cases) and infiltrating ductal BC (90 cases), including infiltrating papillary BC (54 cases) and infiltrating tubular (36 cases) BC. Neoadjuvant antitumor therapy was not performed due to the lack of indications. In the preoperative period, all patients received symptomatic treatment and correction of the comorbid diseases. The group of comparison included axillary lymph nodes of 14 patients with the lethal outcome (aged 44.4±10.2 years old) that earlier underwent treatment at the Municipal Clinical Hospital No. 1 and that did not have oncologic diseases and signs of surgery or injuries of the breasts.
The study was performed according to the ethical principles of studies that involve humans (Helsinki Declaration).
Axillary lymph nodes were fixed in a 10% formalin and processed according to standard methods for inclusion in paraffin. Sections were stained with hematoxylin and eosin and according to van Gieson. For immunohistochemical assessment of angiogenesis development, monoclonal antibodies to CD34 antigen (Dako reagents kit) were used. Sections were processed in accordance with the manufacturer's recommendations (Dako, Denmark). Stained sections were examined in Axioimager M1 light microscope (Zeiss, Germany).
The morphometric analysis of the structural components of the axillary lymph nodes was performed at magnification x10 (test field – 1,400,000 µm2) and x20 (test field – 350,000 µm2) and computer software for the morphological module Axiovision (Zeiss, Germany). On each section, 5 measurements were performed. The volume density of CD34-positive structures was determined throughout the organ and separately in the cortex and medulla, marginal sinus, cortex plateau, paracortex; the volume density of connective tissue was assessed in the same way (when stained according to van Gieson).
Statistical processing of the results was performed with MS Excel 7.0 (Microsoft, USA). The mean values, the error of the mean and Pierson’s correlation coefficient (for the evaluation of the correlations between the parameters of vascularization and sclerotic transformation, and between the stage of BC and the level of development of the connective tissue or parameters of vascularization in the whole lymph nodes and in their compartments) were calculated. Intergroup changes were calculated by Student’s t-test. The differences were statistically significant at p<0.05.
RESULTS
In the axillary lymph nodes of women from the comparison group, the stroma was presented as moderately expressed connective tissue that was identified in the cortex and medulla, mainly along the vessels (Fig. 1a). Small connective tissue layers were also detected around the lymphoid nodules. Moderate parenchyma edema associated with post-mortem alterations was observed. The organ capsule was unevenly pulled.
a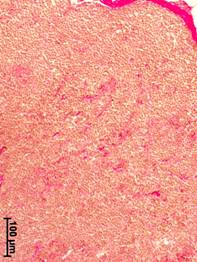 b
b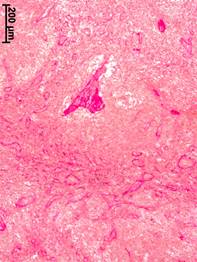 c
c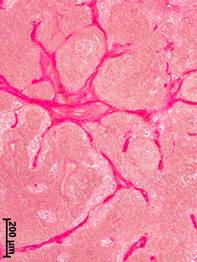
Fig. 1. Morphological changes in the axillary lymph nodes in patients with different stages of breast cancer. Van Gieson stained.
a – cortical substance, no breast cancer; b – medullary substance, stage II; c – medullary substance, stage III
In the axillary lymph nodes of patients with stage I of BC, an insignificant thickening of the capsule (volume density – 1.55±0.60%, in the group of comparison – 1.36±0.49%) and its uneven teasing were observed. The cortex contained minor aggregates of collagen fibers, especially, along the vessels. Connective tissue layers around lymphoid nodules were slightly thickened. The nodules themselves did not contain collagen fibers. In the medulla, sclerotic processes were more expressed than in other compartments. In the medullary cords and trabeculae, a thin collagen network was formed that, in some cases, covered numerous cellular elements.
In patients with stage II of BC, the capsule of axillar lymph nodes was thickened relative to the group of comparison by 36% (volume density – 1.85±0.67%). In the cortex, the growth of collagen fibers was observed not only in trabeculae along the intermediate sinuses but also directly in the lymphoid parenchyma. Thickened bundles of collagen fibers appeared around lymphoid nodules. Signs of sclerosis (a thin network of collagen fibers) were also registered in the nodules. A significant sclerotic transformation was observed in the medullary cords and trabeculae (Fig. 1b). Medullary sinuses contained multilayer fibrous structures that had vascular contours and dendritic shape and reflected the processes of vascular malformation caused by the tumor process.
In patients with stage III of BC, the most significant thickening was observed in the capsules of axillary lymph nodes (volume density – 4.2±0.84%) – by 3 times in regards to the group of comparison and by 2.7 times in regards to stage I of BC (p<0.05). In the cortex, wide layers of the connective tissue were formed that were located primarily subcapsularly and along the blood vessels and lymphatic sinuses. Often, nest-like aggregates of multilayer concentric bundles of collagen fibers were registered. Pronounced sclerotic transformations in the central and peripheral areas of the lymphoid nodules were observed. The most significant sclerotic transformation was registered in the medulla of the axillary lymph nodes. Expansive connective tissue interlayers were formed along the medullary cords and trabeculae (Fig. 1c). In some cases, fragmentation of medulla by connective tissue occurred; one could speak of the formation of a coarse-connective tissue framework.
The morphometric analysis showed that along with the progressing of BC, there was an increase in the volume density of the connective tissue in the axillary lymph nodes. The highest values were observed in patients with stage III (46.4±2.07%). In patients with stage II, it was 28.0±3.65%. In patients with stage I, it did not differ significantly from the group of comparison (3.6±1.76 and 2.64±1.39%, respectively) (Table 1). An increase in the volume density of the connective tissue was observed in all the sections of the lymph nodes. The most significant increase was registered in the cortical substance and cortex plateau (by 24 and 26 times, respectively, p<0.05).
Table 1
Volume density (%) of connective tissue in different compartments of the axillary lymph nodes in patients with different stages of breast cancer (M±m)
|
Compartments |
Group of comparison |
Stages of breast cancer |
||
|
I |
II |
III |
||
|
Whole lymph node, |
2.64±1.39 |
3.60±1.76 |
28.01±3.65*,# |
46.42±2.07*,# |
|
including: |
||||
|
cortex |
0.78±0.30 |
1.35±0.75 |
13.11±2.41*,# |
19.21±1.30*,# |
|
medulla |
1.86±0.56 |
2.25±1.55 |
14.90±2.06*,# |
27.21±2.77*,# |
|
Cortex, |
||||
|
including: |
||||
|
cortex plateau |
0.21±0.03 |
1.0±0.33 |
5.75±1.16*,# |
5.60±0.89*,# |
|
paracortex |
0.57±0.24 |
0.35±0.19 |
5.75±1.53*,# |
7.20±1.10*,# |
|
lymphoid nodules without germinal center |
0 |
0 |
0 |
1.80±0.64*,# |
|
lymphoid nodules with germinal center |
0 |
0 |
1.61±0.79 |
4.61±0.67*,# |
|
Medulla, |
||||
|
including: |
||||
|
medullary cords |
1.00±0.14 |
1.00±0.18 |
5.70±1.34*,# |
6.80±1.48*,# |
|
medullary sinuses |
0.86±0.25 |
1.25±0.57 |
9.15±0.66*,# |
20.41±1.97*,# |
Note: * – p<0.05 relative to the group of comparison, # – p<0.05 relative to stage I of breast cancer.
It should be noted that there were no connective tissue elements in the nodules without a germinal center and in the mantle zone in the group of comparison and patients with stages I and II of BC. There were no such elements in the nodules with a germinal center and mantle zone in the group of comparison and stages I and II of BC (Table 1). Patients with stage II of BC had minor layers of fibrous connective tissue only in the germinal centers of single organs. However, at stage III, the signs of sclerosis were observed in all the nodules of all the lymph nodes.
The volume density of the connective tissue in the medullary cords in patients with stages II and III increased by 5.7 and 6.8 times, respectively, relative to the group of comparison and stage I (Table 1). In the medullary sinuses, this parameter at stage III increased by 24 and 16 times, respectively, in regards to the group of comparison and stage I (p<0.05).
The progressing of BC was associated with an increase in the volume density of vessels (CD34-positive structures) in the axillary lymph nodes (Fig. 2). The value of this parameter in patients with stage III increased by 12, 13, and 2 times, respectively, in regards to the group of comparison, stage I and II of BC (Table 2).
a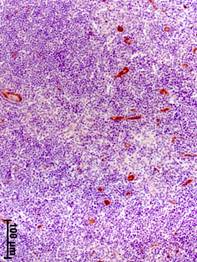 b
b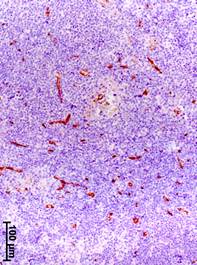 c
c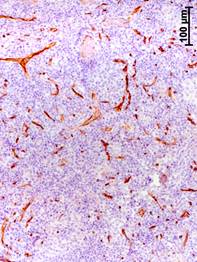
Fig. 2. Immunohistochemical detection of CD34 – positive structures in axillary lymph nodes at different stages of breast cancer.
a – stage I; b – stage II; c – stage III
Table 2
Volume density (%) of vessels (CD34-positive structures) in different compartments of the axillary lymph nodes in patients with different stages of breast cancer (M±m)
|
Compartments |
Group of comparison |
BC stage |
||
|
I |
II |
III |
||
|
Whole lymph node, |
3.00±0.66 |
2.91±0.65 |
18.76±1.38*,# |
36.84±1.92*,# |
|
including: |
||||
|
cortex |
1.29±0.27 |
1.20±0.32 |
9.01±1.38*,# |
15.62±0.89*,# |
|
medulla |
1.71±0.25 |
1.71±0.53 |
9.75±1.37*,# |
21.22±2.17*,# |
|
Cortical substance, |
||||
|
including: |
||||
|
cortex plateau |
0.86±0.16 |
0.95±0.26 |
3.85±0.54*,# |
5.01±0.71*,# |
|
paracortex |
0.43±0.11 |
0.25±0.04 |
4.15±0.81*,# |
5.40±1.34*,# |
|
lymphoid nodules without germinal center |
0 |
0 |
0 |
1.40±0.35*,# |
|
lymphoid nodules with germinal center |
0 |
0 |
1.01±0.26 |
3.81±0.79*,# |
|
Medulla, |
||||
|
including: |
||||
|
medullary cords |
0.57±0.16 |
0.70±0.20 |
3.30±0.57*,# |
5.21±1.11*,# |
|
medullary sinuses |
1.14±0.27 |
1.01±0.36 |
6.45±0.66*,# |
16.01±1.58*,# 3 |
Note: * – p<0.05 relative to the group of comparison, # – p<0.05 relative to stage I of breast cancer.
The volume density of vessels in the cortex in patients with stage III of BC was higher by 12 and 13 times than in the group of comparison and stage I, respectively, and by higher 73.3% than in stage II (Table 2). In the medullary center, this parameter in patients with stage III was higher by 12, 12.5 and 2 times than in the group of comparison, stages I and II. In the paracortex, the volume density of vessels in patients with stage III of BC was higher by 12 and 22 times, respectively, than in the group of comparison and stage I. It should be noted that there were no vessels in the lymphoid nodules with and without germinal centers in women from the group of comparison (without BC) and in patients with stage I. In patients with stage II of BC, small vessels with sclerosed walls appeared only in the germinal centers of single organs. However, in patients with stage III, they were observed in all the lymph nodes (Table 2). The volume density of vessels in the medullary sinuses of the axillary lymph nodes significantly increased. In patients with stage III, it was higher by 14, 16, and 2.5 times, respectively, than in the group of comparison, and stages I and II.
The results of the morphometric analysis indicate that the processes of vascularization and sclerotic transformation of lymph nodes and their compartments develop simultaneously along with the progression of tumor growth. Direct positive correlations were established between the volume density of vessels and the volume density of the connective tissue in the axillary lymph nodes. In patients with stage I, it was r=0.747 (p<0.05), stage II – r=0.707 (p<0.05), and stage III – r=0.664 (p<0.05), i.e. in all the cases, a significant positive correlation was revealed. In the group of comparison, a weak negative correlation was revealed (r=–0.298). Negative correlation in women without BC could be explained by the fact that the processes of formation of the connective tissue were not associated with the processes of angiogenesis or that gradual organ sclerosis (for example, aging) was not associated with the formation of new vessels.
The analysis of the correlations between the stage of BC and the volume density of the connective tissue showed that this correlation was direct and strong: r=0.981 (p<0.05). A similar correlation was revealed separately for the cortex (r=0.953, p<0.05) and medulla (r=0.976, p<0.05). Thus, along with the tumor progression (stage increase), an increase in the volume density of the connective tissue is observed in the whole organ and separately in the cortex and medulla. The intensity of the sclerotic transformation in these areas is similar.
A strong positive correlation was revealed between the stage of BC and the volume density of vessels in the axillary lymph nodes (r=0.984; p<0.05). Similar consistency was established for the cortex (r=0.978; p<0.05) and medulla (r=0.965; p<0.05).
Intensification of lymph node sclerosis with tumor progression could be associated with a longer and more expressed peritumoral inflammatory process, which can contribute to the intensification of destructive processes and, as a result, lead to substitutive sclerosis. Connective tissue hyperplasis along the sinus system of lymph nodes and the sclerotic transformation of vascular walls contribute to the disturbances of metabolism and development of hypoxia in significant areas of the lymphoid parenchyma. In such conditions, an intensification of the production of different proangiogenic cytokines that stimulate the differentiation of multipotent stromal cells into endotheliocytes can be observed, as well as an intensification of neoangiogenesis for the improvement of blood supply of the organ and liquidation of hypoxia [13, 14]. It is also possible that proangiogenic cytokines [15] produced by the tumor penetrate axillary lymph nodes and stimulate the growth of vessels in all their structural compartments, including lymphoid nodules. This results in the appearance of a network of newly formed vessels that have a narrow lumen and thing one-layer wall. Then in such vessels, the lumen expands, and membranes are formed, in particular the adventitia. The outer membranes of adjacent vessels merge over time, and connective tissue layers, which are formed as a result of detritus from the site of tumor development, also expand and merge. Thus, the severity of sclerotic changes increases simultaneously with angiogenesis.
CONCLUSIONS
In the axillary lymph nodes, the volume density of the connective tissue progressively increases with the stage of BC (stage III – by 18 and 13 times, respectively, in regards to the group of comparison and stage I). It should be noted that in some cases, the layers of the connective tissue in patients with stage II of BC appeared even in germinal centers of the lymphoid follicles. Sclerosis progressed quickly in the paracortex and medullary sinuses, where the relative area of the connective tissue increased ten-fold.
Along with the progressing of the pathological process in patients with BC in the axillary lymph nodes (stage III – by 12 and 13 times, respectively, in regards to the group of comparison and stage I) and in all their compartments, a quick and significant increase in the volume density of the newly formed vessels that appear even in the lymphoid nodules was observed.
In the axillary lymph nodes of patients with BC, a strong positive correlation between the volume density of vessels and volume density of the connective tissue was revealed. In women without BC, this correlation was weak and negative. The obtained results showed that the processes of angiogenesis and sclerotic transformation in the lymph nodes in patients with BC were parallel and had the same intensity. Probably, these processes mutually potentiate each other.
Strong positive correlations were revealed between the stage of BC and the volume density of the connective tissue and between the stage of BC and the volume density of the newly formed vessels in the axillary lymph nodes. It can be suggested that in non-metastatic lymph nodes, the angiogenesis intensifies and sclerotic transformation enhances in patients with BC along with the progression of the tumor growth. At the same time, the identification of significant sclerosis in the lymph node cannot be taken as a definite symptom of malignant tumor in the region of lymph collection, because the appearance of connective tissue can be associated with many other reasons. However, simultaneous significant intensification of neoangiogenesis can be one of the signs of a long-term peritumoral inflammatory process, which should be accounted for during the evaluation of the severity of systemic signs of the malignant process and the application of adequate schemes of rehabilitation of patients.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
The authors declare no conflict of interest



