INTRODUCTION.
The treatment of oncologic brain pathologies is one of the main tasks in modern highly technological medicine. In the world medical practice, boron neutron capture therapy (BNCT) is seen as a promising experimental method of treatment for brain glioblastoma [1; 2]. The method is based on the selective accumulation of non-radioactive isotope boron-10 in the tumor cells and further irradiation with the neutrons. The capture of neutrons by boron-10 induces nuclear reaction with the expression of energy in the tumor cell, which leads to its death [3; 4].
The present study was aimed to analyze the effect of BNCT using L-p-boron phenylalanine (BPA) and sodium borocaptate (BSH) as target boron delivery agents on the growth of tumor in animal model in vivo.
MATERIALS AND METHODS
The study was performed at the facilities of the Center of Genetic Resources of Laboratory Animals of the SPF vivarium of the Institute of Cytology and Genetics SB RAS. All the experiments on animals were approved by the interdisciplinary committee on bioethics and corresponded to the Guide for the Care and Use of Laboratory Animals issued by US NIH No. 85-23, revised in 1985.
Tumor orthotopic xenograft in animals. U87 cell line (human glioblastoma) was cultivated in the medium DMEM-F12 (Biolot, Russia) with 10% fetal bovine serum (Gibco, USA) and gentamicin 50 µg/ml (Belmedpreparaty, Republic of Belorussia) in a CO2-incubator at 37 °C with a supplement of 5% CO2. Eight-week immune-deficient male SCID mice were used as experimental models. Intracranial inoculation of 500 thousand U87 cells was performed with further MRI control (tomograph BioSpec 117/16USR) for the verification of the tumor presence and the of its size.
Tumor heterotopic xenograft in animals. In the group of eight-week-old Nu/J line male mice, subcutaneous injection of 10 mln of U87 cells was performed. The volume of the tumor was controlled by the measurement of its length and width.
L-p-boron phenylalanine (BPA) and sodium borocaptate (BSH) enriched with 10B isotope were used for target boron delivery agents (Katchem, Czech Republic). The solution of BPA was prepared at a dose of 350 mg/kg of body weight. For the dilution of the drug, D-fructose was used in the excess molar quantity. The concentration of boron in the solution was 4.2 mg/ml. The drug was injected in the retroorbital sinus at a dose of 2.5 µl/g 2 hours before the radiotherapy. The solution of BSH was prepared in the 0.9% saline solution at a dose 100 mg/kg. The concentration of 10B in the solution was 17 mg/ml. The drug was injected in the retroorbital sinus at a dose of 4 µl/g 1.5 hours before the radiotherapy.
Irradiation of mice with intracerebral tumor xenografts. BNCT was performed on 24th day after inoculation of tumor cells. The mice were distributed into four groups, six animals in each group. Group I received BNCT BPA, Group II – BNCT BSH, animals from Group III were exposed to the radiation with epithermal neutrons without boron agents, Group IV was used as a control. Animals from all groups were anaesthetized with an intraabdominal injection of domitor and zoletil. The animals were transported in the foam thermo-isolated container. The radiation was performed using the accelerator based epithermal neutron source at Budker Institute of Nuclear Physics SB RAS [5]. The influence of neutron radiation on cell cultures that were incubated in the boron-containing medium was studied [6]. The mice were positioned radially with their heads to the center of a special container that which covers animal body with a layer of lithium polyethylene. During the radiation, animals from the control group were in container with adequate access of oxygen and optimal environmental temperature conditions. Overall radiation time was 2 hours 46 minutes. During the experiment the following parameters: the current and energy of the proton beam and the density of the neutron beam were monitored. Dosimetry based on the activation of gold was performed. The overall integral current was 5.46 mA/hour. According to the calculations, animals from Group BNCT BSH were exposed to 28.8 Gy-Eq, BNCT BPA – 12.2 Gy-Eq, the group that was radiated with neutrons – 4.7 Gy-Eq. Induced radioactivity was 4 µSv/hour.
Irradiation of mice with subcutaneous tumor xenografts. Animals were distributed into two groups by tumor volume: Group I underwent BNCT BSH and Group II was used as control. The radiation was performed on 20th day after subcutaneous injection of tumor cells. Mice were placed in plastic containers (two animals per container). Four containers were located on a rotating stand as a square with side of 20 cm length. There was a Plexiglas cylinder (200 mm in diameter, 36 mm thick) placed between the target and the containers. Adequate access of air and optimum temperature conditions were provided. Overall radiation time was 2 hours 46 minutes. Overall current integral was 3.48 mA/hour. Normal tissue was exposed to the radiation at a dose of 3.2-4.0 Gy-Eq, tumor tissue – 5.0-6.0 Gy-Eq.
The observation of the therapeutic effect and the analysis of the results in animals with intracerebral tumor xenografts were performed daily. The examination of animals included evaluation of their activity, development of paresis, coordination and body weight. The evaluation of the tumor size was performed using T2 weighted images by the method of TurboRARE (Rapid Imaging with Refocused Echoes). The parameters of impulse sequence of the method were TE = 33 ms, TR = 1500 ms; the parameters of the images: size 2.5 × 2.5 cm, matrix 512 × 512 pixels, pixel size 0.45 × 0.45 µm; section thickness – 0.5 mm; distance between sections – 0.5 mm; the number of sections – 15; the orientation of the section was axial, the overall scanning time was 3 minutes. An example of MRI of a mouse with an intracerebral tumor xenograft is presented in Fig. 1.
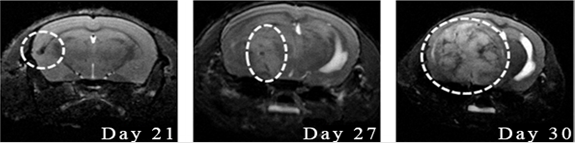
Fig. 1. MRI visualization of the dynamics of intracerebral tumor U87 growth: T2-weighted images, coronal projection, Day 21, 27 and 30 after tumor implantation. MRI signs of progressive tumor growth
Statistical processing of the data was performed using the software package Microsoft Excel 2010. Categorical variables were presented in percent. The comparative analysis of the values in the test and control groups was conducted using the Mann-Whitney test. The results were significant at p = 0.05.
RESULTS
The results of in vivo study showed that the survival of animals increased by 30% in comparison with the control group that received BSH (Mann-Whitney test) (Fig. 2).
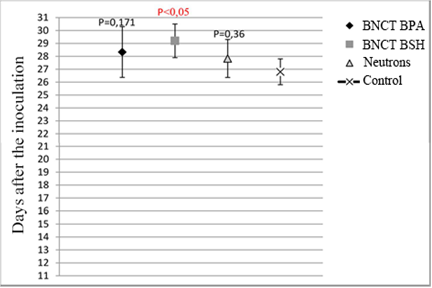
Fig. 2. Median survival in experimental groups after the BNCT BSH and BNCT BPA
Experimental results also revealed the changes in the tumor volume on 30th day Thus, the mean volume of the tumor in groups that received BNCT was smaller than in the group that was exposed to radiation without boron delivering drugs (Fig. 3).
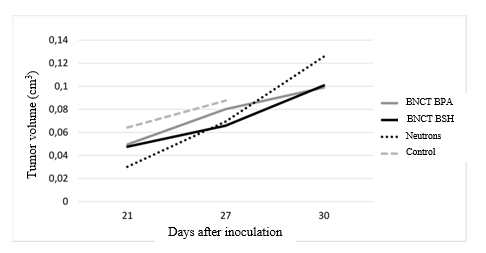
Fig. 3. Dynamics of the tumor growth in four groups
The data analysis allowed to calculate the rate of the lethality of animals in the studied groups. In general, animals that received boron delivery drugs and animals that were exposed only to neutrons lived 2 days longer than in the control group (Fig. 4).
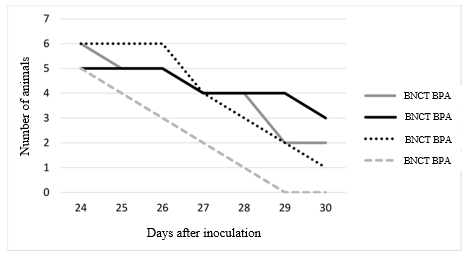
Fig. 4. The rate of lethality of animals in experimental groups
The evaluation of the therapeutic response and the analysis of the results obtained in animals with subcutaneous tumor xenografts.
The observation of the therapeutic effect of BNCT showed that tumor in mice from control group grew significantly faster in comparison with the animals that received BNCT. On 4th day after irradiation, there was a sharp decrease in the tumor focus volume in the group that received BNCT (Fig. 5).
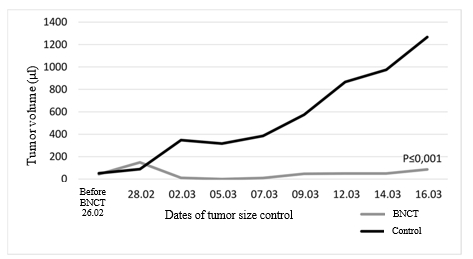
Fig. 5. Dynamics of tumor volume in Nu/J mice with U87 implanted subcutaneously
The results of observation of the BNCT effect are presented in Fig. 6. Pictures A, C, E show mice with tumor up to 100 µl before BNCT. Pictures B, D, F show mice with tumor volume less than 200 µl 23 days after BNCT. Picture G shows a mouse from the control group with the tumor volume 52 µl. Picture H shows a mouse that was not injected BSH, 23 days after the radiation: large subcutaneous tumor of 1449 µl volume with an expressed vascular network is well visualized.
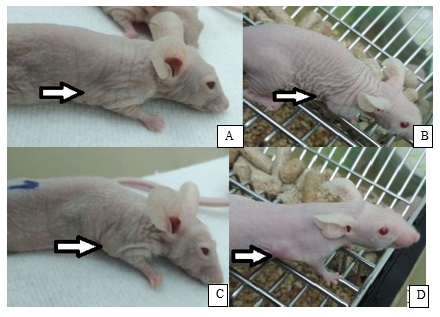
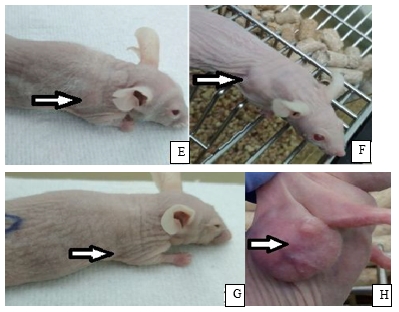
Fig. 6. Nu/J mice with subcutaneous U87 tumor: A, C, E, G – before radiotherapy; B,D, F, H – 23 days after radiotherapy (see comments below in the text)
Despite the existing technologies, radical surgical treatment, the combination of radio and chemotherapy, the median survival in patients with glioblastoma is around 15 months [7]. In clinical studies of BNCT, around 2000 patients took part worldwide. The accumulated data demonstrates the positive influence of this therapy on the survival of patients with recurrent and newly diagnosed glioblastoma. According to Kageji et al. [8], the median survival was 19.5 months in patients that were exposed to BNCT in combination with standard types of therapy, and two-year, three-year, and five-year survival was 31.8, 22.7, and 9.1%, respectively. In the study by Yamamoto et al. [9] median survival was 25.7 months after BNCT of primarily diagnosed glioblastoma. However, further studies are required for the establishment of optimal therapeutic protocols and development of accelerator based neutron sources that can simplify the installation of the equipment for BNCT at clinics and provide the wide implementation of this method of treatment [10].
Accelerator based epithermal neutron source was developed at Budker Institute of Nuclear Physics SB RAS completely meets the requirements of BNCT [5]. The performed studies on the neutron source show a relative safety and therapeutic effectiveness of the beam [2; 6]. However, taking into account the originality of the setting, further in vitro and in vivo studies are necessary for the evaluation of the biological effects. This will provide scientific grounds for the implementation of this method in clinical practice. For evidence-based medicine, the most informative experiments are those that involve models of human tumors in laboratory animals. Human tumors can be successfully transplanted to mice and rats with nu mutation [11].
Until now, the modeling of the effects of neutron capture therapy in animals was performed using nuclear reactors and boron delivery agents BSH and BPA. The first experiments with BSH as a delivering agent for BNCT were performed in 1967 [12]. The potential of this drug was evaluated in mice with an implanted subcutaneous tumor of mice ependymoblastoma. The overall dose of BSH varied from 140 to 175 mg of boron per gram of body weight. In 1968, Hatanaka et al. [13] initiated the study of therapy in mice with implanted C57BL methylcholanthrene-induced sarcoma. The variable doses of BSH were injected intraperitoneally. The animals were divided by the time and predicted dose of the neutron radiation. The studies showed that in 7 mice out of 13, after a session of BNCT, there were no signs of tumor growth or therapy-associated toxicity. Based on this data, Hatanaka initiated the clinical investigation in patients with highly malignant gliomas. There were also experiments on combining BNCT with radiation therapy in rats with implanted glioma F98 [14].
The studies on the effectiveness of neutron capture therapy were also performed in Russia. Thus, scientists from National Research Nuclear University MEPhI performed experiments on dogs aged 9 to 15 years old with histologically verified diagnosis of melanoma of the oral mucosa. BPA and Dipentast were used as boron-delivering agents [15]. In the group of animals that received gadolinium neutron capturing therapy, in 70% of cases, complete regression of the tumor was observed within 30-45 days. In 14% of animals, the tumor recurrence was registered in 1 month. The life expectancy was 4-6 months. At the same time, in the group of animals that received BNCT, complete regression of the tumor was registered in 95% of cases. In 20% of animals, the tumor recurrence was revealed in 3-4 months. The life expectancy was 8 months. In the group that received gamma-therapy, 90% of dogs had tumor recurrence in 1 month after the therapy and metastases – in 2-3 months. The life expectancy was 3 months.
The method of modeling tumor orthotopic and heterotopic xenograft in immune-deficient mice using U87 human glioblastoma cell line was successfully developed. The results of present study on BNCT in vivo at innovative accelerator revealed two facts. First, BNCT reduced and, in some cases, completely inhibited the tumor growth. Second, median survival in experimental groups after BNCT with BSH was longer than in groups that received BNCT with BPA and control group.
CONCLUSIONS
The clinical experiment in vivo performed at accelerator based epithermal neutron source at Budker Institute of Nuclear Physics SB RAS confirmed safety and effectiveness of BNCT for human glioblastoma. Overall results of the study obtained on the experimental tumor models up to 100 µl permits the development of the program of investigations in vivo on subcutaneous tumor models up to 500 µl. The performed study revealed a tendency toward a reduction or inhibition of tumor growth in group that received BNCT. This confirms the prospects of this method of treatment and the technology of neutron generation. We believe that further studies should be performed involving more experimental animals.
FINANCIAL SUPPORT AND SPONSORSHIP
The study was supported by The Russian Foundation for Basic Research within the scientific project No. 18-29-01007.
CONFLICTS OF INTEREST
The authors declare no conflict of interest



