INTRODUCTION
Collagen fibrils are the most typical components of fibrous tissue in the human or animal bodyand are characterised by complicated structure. Collagen fibrils consist of the collagen protein, which determines the properties of these structures. At present, there are 28 known types of collagens that differ in polypeptide chain structure, and each type is expressed by a specific gene. Type I collagen is found in solid and soft tissues. It is the main protein of bones, derma, and tendons. Type II collagen is localised primarily in hyaline cartilaginous tissue, the vitreous body, and the pulpal nucleus of the intervertebral disc [1].
In the last few years, there were numerous publications on the reaction of the extracellular matrix to tumour progressionin patients [2; 3]. It has been demonstratedthat collagens can directly influence the proliferation and differentiation of the mammary epithelium [4]. It is also reported that type I collagen acts as a physical barrier to the migration of cells and prevents the proliferative activity of normal and cancerous cells [5]. Degradation of type I collagen can play a crucial role in the acquisition of invasive characteristics by breast cancer [6; 7].
There is a need for further detailed investigation into the mechanisms behind theinfluence of the extracellular matrix – and in particular, collagens of different types – on tumour progression.
The aim of the study was to characterise the distribution of type I and II collagens in the tumour tissue of invasive breast carcinoma of no special type or breast fibroadenoma.
MATERIALS AND METHODS
The study involved samples of surgically resected tumour specimens obtained from 39 patients ofaverageage 59 years (range 55.0–66.8) with invasive breast carcinoma of no special type. Besides, the study included surgical samples obtained from 11 patients of average age 48 years (range 28.0–56.8) with breast fibroadenoma.
The study protocol conformed to theguidelines for experimental research on human subjects in accordance with the Declaration of Helsinki and was approved by the ethics committee of Novosibirsk State Medical University of Russian Ministry of Health . Written informed consent was obtained from each patient (or a legal guardian) before the study.
Breast tumour samples were fixed in a 10% solution of formalin. Seven-micrometre-thick serial sections were prepared from the wax-embedded material. For the evaluation of stainingproperties, collagen fibrils were stained with Van Gieson picrofuchsin.
Immunohistochemical analysis of type II collagen expression was performed on wax-embedded sections of tumour specimens by an indirect ABC-peroxidase method by means of theVectastain Universal Elite ABC Kit (Vector Laboratories, cat. No. PK7200). To identify type II collagen, a mouse monoclonal antibodyto COL2A1 (M2139, cat. No. sc52658, Santa Cruz Biotechnology, Inc.)wasused at dilution 1:100 according tothe recommendations of the manufacturer. For the identification of type I collagen, immunohistochemical analysis was performed on the tumour sections with the Vectastain Universal Elite ABC Kit (Vector Laboratories, cat. No. PK-7200). For this purpose, a mouse monoclonal antibodyto COL1A (COL-1) wasemployedat a dilution of 1:100 in accordance with recommendations of the manufacturer (cat. No. sc-59772, Santa Cruz Biotechnology, Inc.). In both cases, diaminobenzidine served as a chromogenic substrate (the solution was prepared ex tempore from the components of the kit ImmPACT DAB (cat. No. SK-4105, Vector Laboratories).
The morphometric analysis was performed for each patient on eight micrographs with a total area of 95,587 µm2. The analysis of the relative area occupied by type I and II collagens andevaluationofthe specific staining intensity were performed inthe ImageJ 1.42gsoftware (National Institutes of Health, USA). For assessment of the stainingproperties of collagen fibrils, RGB-model analysis was performed by means of an additional module: Measure RGB.
Statistical analysis was performed in the SPSS software v17.0 for Windows. The independent groups were compared by the Kruskal–Wallis test with a further intergroup comparison of the parameters by the Mann–Whitney test. The differences were assumed to be statistically significant at p < 0.05. The obtained data were presented as a median (Me) and the interquartile range (Q1; Q3).
RESULTS
The majority of tumour samples of invasive breast carcinoma of no special typecontainedareas in the tumour with significant dissociation of collagen fibres, counter smearing, and segmentation. Some tumourscontained areas with lumpy degradation of collagen fibrils. Nearly all the tumour samplesin this group featurednumerous vast areas of altered physicochemical properties of collagen fibrils manifested as the change in their stainingproperties (Figure 1).
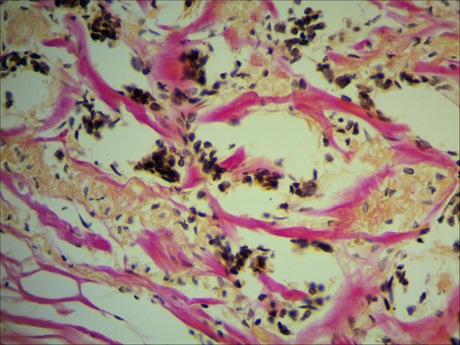
Fig 1. Changes in the stainingproperties of orange-stained collagen fibres in a tumour sample of invasive breast carcinoma of no special type. Van Gieson picrofuchsin staining,magnification ×400.
A morphological analysis of the tumour samples from patients with fibroadenoma did not reveal areas of degenerative alterations of collagen fibrils. Moreover, the bundles of collagen fibrils were found to be tightly connected. Unlike tumour samples from the other group, thefibroadenoma samples did not show a change in the stainingproperties of collagen (Figure 2).
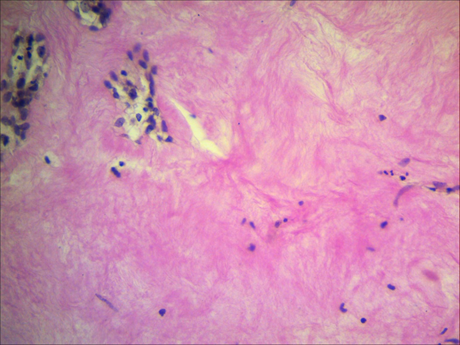
Fig. 2. Preservation of the stainingproperties of collagen fibres in a patient with breast fibroadenoma. Van Gieson picrofuchsin staining,magnification ×400.
The results of the RGB-model analysis are presented in Table 1. They revealed a shift in the staining of collagen fibres to the orange rangeof the visible spectrum in the group of tumour samples from invasive breast carcinoma of no special type. These alterations indicate that changes in the stainingproperties of collagen fibrils can be associated not only with their degradation but also with the synthesis of collagen of another type.
Table 1.
Results from the RGB model of photomicrographs of biopsy samples of benign and malignant breast tumours (the values of channels are presented in conventional units [CU; Me (Q1; Q3)].
|
Channel/group |
invasive breast carcinoma of no special type |
breast fibroadenoma |
|
red |
197.7 (190.3; 205.1) p1-2 = 0.018 |
188.0 (176.1; 191.9) |
|
green |
146.5 (129.4; 159.4) p1-2 = 0.0001 |
98.4 (85.6; 112.5) |
|
blue |
186.4 (175.0; 194.0) p1-2 = 0.361 |
177.5 (162.7; 186.2) |
The immunohistochemical experiment revealedthat the main type of collagen in these breast tissues is type I collagen (Figure 3).
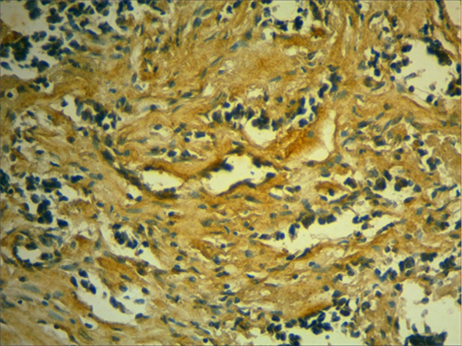
Fig. 3. Type I collagen in the stroma of a patient with invasive breast carcinoma of no special type. Immunohistochemical identification of type I collagen with subsequentadditional hemalum staining of the nuclei,magnification ×400.
Totest whether another type of collagen is synthesised that differs from the collagen found in the healthy breast, we performed immunohistochemical staining of the biopsy samples for type II collagen. Figure 4 indicatesthat some stroma cells expressed type II collagen,which is not typical for this tissue type. As a consequence, we noted the formation of collagen fibrils atypical for this tissue. The results from the morphometric analysis of the relative area occupied by type I and II collagens and the intensity of the specific staining are presented in Table 2. Thesedata uncoveredan increase in the relative area occupied by type II collagen and in the intensity of its staining in the tumour samples ofinvasive breast carcinoma of no special type in comparison with breast fibroadenoma.
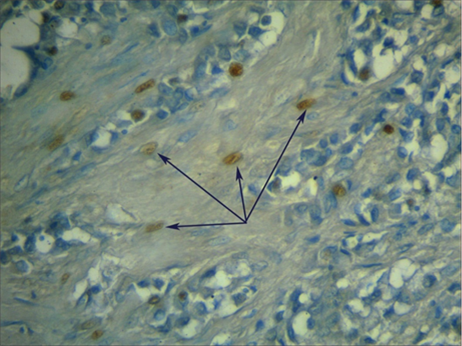
Fig. 4. Expression of type II collagen in tumour-associated fibroblasts in atumorsample of invasive breast carcinoma of no special type. Immunohistochemical identification of type II collagen by additional hemalum staining of the nuclei(magnification ×600).
Table 2
Morphometric analysis of the relative area occupied by type I and II collagens and the intensity of the specific staining [Me (Q1; Q3)].
|
Parameter/group |
invasive breast carcinoma of no special type |
breast fibroadenoma |
|
Relative area occupied by type I collagen, (%) |
28.0 (22.9; 31.4) p1-2 = 0.014 |
31.8 (28.4; 36.9) |
|
Intensity of staining of type I collagen, (CU) |
56.8 (47.3; 64.7) p1-2 = 0.023 |
71.9 (55.6; 84.1) |
|
Relative area occupied by type II collagen, (%) |
49.2 (44.1; 55.3) p1-2 = 0.00001 |
26.2 (24.0; 30.1) |
|
Intensity of staining of type II collagen, (CU) |
86.7 (81.1; 92.4) p1-2 = 0.00001 |
50.8 (43.1; 71.3) |
The obtained data suggestedthat the changes in the stroma of fibroadenoma were mediated byenhanced synthesis of type I collagen.The lattercorresponds to the main collagen of loose connective tissue in the breast. Breast tissues of patients with nonspecific adenocarcinoma undergo a significant transformation of desmoplastic stroma: along with foci of dissociation and degradation of collagen fibrils, there are areas containingmesenchymal cells that stronglyexpress type II collagen,which is atypical for this type of tissue. Thus, it can be suggested that patients with breast cancer have not only atypical epithelial cells but also specificstromal components inthe tumour.
The extracellular matrix is an important regulator of breast cancer progression. Matrix proteins that are produced in patients with breast cancer include fibrillar collagens, fibronectin, specific laminins, and proteoglycans as well as matrix cellular proteins. Numerous proteins of the extracellular matrix play an important role in the progression of breast cancer and metastases [8; 9]. It is believed that fibroblasts associated with cancer performimportant functions in the progression, survival, metastasis, and invasiveness of cancer via the secretion of variousgrowth factors, cytokines, and chemokines and throughdegradation of extracellular-matrix proteins [10; 11].This observationsuggests thatthese fibroblastsare a special cellular population different from regular fibroblasts.
CONCLUSIONS
This study revealed some specific featuresof the stroma in malignant orbenign tumoursof the breast. Ouranalysis of the RGB model inphotomicrographs of the biopsy samples uncovereda significant increase in the red and green components.Forthe group of patients with invasive breast carcinoma of no special type,this findingmeansashift in collagen fibril staining to the orange rangeof the visible spectrum. Suchchanges in the physicochemical properties of collagen fibrils can be causednot only by their degradation but also by the synthesis of type II collagen by tumour-associated fibroblasts.The latter phenomenon is atypical for the loose connective tissue of the breast. Along with type I collagen, someof the stromal cells started to secrete type II collagen,which has a different structure of the polypeptide chain. In these cells,such a changeindicates expression of the genes that are typical for cartilaginous tissue, i.e. these cells acquired the properties of osteoblasts. Thus, it can be proposedthat patients with invasive breast carcinoma of no special type have not only atypical epithelial cells but also some specific stromal tumour component, which can be regardedas an additional diagnostic feature of this tumour’s malignancy.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
The authors declare no conflict of interest



