Aims
Study objective: to identify molecular and morphological effects of preoperative radiotherapy in rectal cancer.
Materials and Methods
We analysed data from 45 patients who underwent radiotherapy during the perioperative period. DNA (DNA distribution over the cell cycle phases) was tested in tumour cells of the resected intraoperative tumour tissue (CycleTESTTMPLUS DNA ReagentKit). The DNA index characterised by aneuploid to diploid cell ratio was determined. Therapeutic pathomorphism and change in tumour size induced by preoperative radiotherapy were recorded.
Results
The DNA index test has showed no tumours with DNA index less than 1.0. The index ranged from 1.1 to 1.9. Radiotherapy was associated with a 1.6-fold increase in tumour incidence with DNA index up to 1.5 and a 2.6-fold decrease in tumour incidence with DNA index greater than 1.5. Grade III therapeutic pathomorphism was reported in 70% of cases. The tumour extent decreased 1.5-fold, and the distance from the anus to the lower edge of the tumour increased 1.2-fold (р<0.05).
Conclusions
The molecular and morphological effect of radiotherapy for rectal cancer was a reduction in tumour malignancy potential, manifested by varying degrees of therapeutic pathomorphism. Our data suggest that, 6–8 weeks after prolonged radiotherapy, there is a pronounced clinicopathological effect, and the molecular changes already correspond to a non-irradiated tumour by many parameters at this time. Consequently, extending the period between the end of radiotherapy and surgery may result in resumption of tumour growth and reversal of the effect of neoadjuvant therapy. This should be considered when planning combination treatment in patients with rectal cancer.
1. Kit O.I., Gevorkian Iu.A., Soldatkina N.V. et al. Combined surgery for locally advanced colorectal cancer [Kombinirovannye operativnye vmeshatel’stva pri mestno- rasprostranennom kolorektal’nom rake]. Pirogov Russian Journal of Surgery - Khirurgiia. Zhurnal imeni N.I. Pirogova, 2016, no. 11, pp. 42-47, doi: 10.17116/hirurgia20161142-47.
2. Kit О.I., Vodolazhskiy D.I., Gevorkyan Y.A. et al KRAS Gene Mutations and Gender Differences in Colorectal Cancer. International Journal of Bio Medicine. 2015, vol. 5, no.1, pp.11-15, doi 10.21103/Article5(1)_CR2.
3. Kit O.I., Gevorkian Iu.A., Soldatkina N.V. et al. Frequency and spectrum of kras gene mutations in advanced colorectal cancer. Clinical-morphological features [Chastota i spektr mutatsii gena KRAS pri rasprostranennom kolorektal’nom rake]. Molecular medicine - Molekuliarnaia meditsina, 2015, no. 5, pp. 26-29.
4. Ferrari L. Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep (Oxf). 2015, vol. 3, no.4, pp. 277-288.
5. Abrams M.J., Koffer P.P., Leonard K.L. et al. The emerging non-operative management of non-metastatic rectal cancer: A population analysis. Anticancer Research. 2016, vol. 36, no. 4, pp. 1699-1702.
6. Gahagan J.V., Whealon M.D., Phelan M.J. et al. Improved survival with adjuvant chemotherapy in locally advanced rectal cancer patients treated with preoperative chemoradiation regardless of pathologic response. Surgical Oncology. 2020, vol. 32, pp. 35-40, doi 10.1016/j.suronc.2019.10.021
7. Kreis M.E., Ruppert R., Kube R. et al. MRI-Based Use of Neoadjuvant Chemoradiotherapy in Rectal Carcinoma: Surgical Quality and Histopathological Outcome of the OCUM Trial. Annals of Surgical Oncology. 2020, vol. 27, no. 2, pp. 417-427, doi 10.1245/s10434-019-07696-y.
8. Maas M., Dijkhoff R.A.P., Beets-Tan R., Rectal Cancer: Assessing Response to Neoadjuvant Therapy. Magnetic Resonance Imaging Clinics of North America. 2020, vol. 28, pp.117-126.
9. Maeda K., Shibutani M., Tachimori A. et al. Prognostic significance of neoadjuvant rectal score and indication for postoperative adjuvant therapy in rectal cancer patients after neoadjuvant chemoradiotherapy. In Vivo. 2020, vol. 34, no. 1, pp. 283-289, doi 10.21873/invivo.11772.
10. Taylor F.G., Quirke P., Heald R.J. et al. Preoperative magnetic resonance imaging of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. Journal of Clinical Oncology. 2014, vol. 32, no. 1, pp. 34-43, doi 10.1200/jco.2012.45.3258.
11. McCoy M.J. Neoadjuvant chemoradiotherapy for rectal cancer: how important is tumor regression? ANZ Journal of Surgery, 2015, vol. 5, pp. 23-29.
INTRODUCTION
Modern oncology achieved significant success in the treatment of colorectal cancer [1–3] and radiotherapy was included in the combined therapy for colorectal cancer long ago [4, 5]. Numerous studies showed an important role of preoperational radiotherapy in a complex treatment for colorectal cancer. In the USA, from 2004 to 2011, the share of patients that received neoadjuvant radiotherapy increased from 57% to 75%, and the share of patients that received postoperative radiotherapy decreased from 39% to 18% [6, 7]. Similar tendencies were observed in Europe and Russia.
The indication of preoperational radiotherapy leads to a two-fold decrease in the 5-year rate of recurrence-free survival in patients with colorectal cancer (from 10.9% to 5.6%) [8–10]. It was established that the main prognostic sign of the effectiveness of radiotherapy in patients with malignant tumors was the degree of tumor regression. Thus, 3-year recurrence-free survival in patients with Dworak 4 is 95%, Dworak 3 – 82%, Dworak 2 – 64%, and Dworak 1 – only 53% [11]. The achievements in fundamental oncology allow specialists to evaluate the degree of tumor regression at the tissue, cellular, and molecular levels.
The study was aimed to reveal the molecular-morphological effects of perioperative radiotherapy in patients with colorectal cancer.
MATERIALS AND METHODS
The authors analyzed the data obtained from 45 patients with colorectal cancer T3-4N0-1M0 that underwent therapy at the National Medical Research Oncological Center of the Ministry of Healthcare of the Russian Federation. In all the patients, the tumor was characterized as adenocarcinoma (more often, it was moderately differentiated (75%)). The main group included 20 patients that underwent a course of neoadjuvant radiotherapy for a tumor and pathways of metastases (19 sessions 5 times per week with a fraction dose of 2.4 Gy to a total boost dose of 50 isoGy). In the days of therapy, radiomodification with capecitabine (1600 mg2/day) was indicated. Surgical treatment was performed in 7–8 weeks after radiotherapy. The control group included 25 patients that had a contraindication to radiotherapy at the first stage or refused the therapy. A post-operational tumor specimen was sent for pathomorphological study. The degree of therapeutic pathomorphism of the tumor was identified by Lavnikova’s method. Tissue DNA analysis was performed with the CycleTESTTMPLUS DNA ReagentKit (No. 340242, BectonDickinson). The obtained data were statistically processed with the ModFit LT software. The software was used to analyze the ploidy and distribution of cells by the phases of the cellular cycle. Besides, the share of cells with different content of DNA was calculated. The proliferation index was defined as the sum of tumor cells at the phase of S- and (G2+M) of the cellular cycle. Student’s t-test was used for data processing.
The study protocol followed guidelines for experimental investigation with human subjects in accordance with the Declaration of Helsinki and was approved by the ethics committee. Written informed consent was obtained from each patient (or official representative) before the study.
RESULTS
The results of the morphological study showed that the changes in tumors after radiotherapy were characterized by the development of destructive foci. In the main group, the area of necrosis was 36.4±4.2%, the signs of irreversible forms of dystrophy varied from 24% to 68%. In the present study, therapeutic pathomorphism of grades I and IV was not revealed. Therapeutic pathomorphism of grade II was revealed in 25% of patients and grade III – in 70% of patients. It manifested itself as the lack of tumor cells, expressed fibrosis and hyalinosis of the connective tissue, the presence of focal calcification, and gigantic multinucleate cells like foreign matter. Flow cytometry was used to study the quantity of DNAs in tumor cells, their distribution by the phases of the cellular cycle, and proliferative activity of tumor cells under the influence of a course of neoadjuvant radiotherapy. It was proved that the content of DNA in a normal cell was inconstant. It is also known that a tumor is heterogeneous. The tumor growth is provided by an actively proliferating cell fraction. The cells at the phase of synthesis (S) and pre-synthesis (G1) are least sensitive to a radiative effect and cells at the phase of mitosis (M) and pre-mitosis (G2) are most sensitive.
The effect of drugs was different. Some alkylating agents affect the cells in the synthetic and pre-mitosis phase; plant-derived cytostatics – at the phase of mitosis, inhibitors of protein synthesis – in the pre-synthetic phase; and the effect of antimetabolites is observed in the synthetic and pre-synthetic phases. The study of DNA-cytometric parameters of colorectal tumors under the influence of radiotherapy revealed the prevalence of aneuploid tumors (60%) over diploid ones (40%). The share of tumors with a DNA index higher than 1.5 was 16.7%. In the control group, the share of aneuploid tumors was 64%. The majority of tumors were heterogeneous, i.e. they contained both aneuploid and diploid cells. The main factor that defines the biological behavior of the tumor is the characteristics of the mean content of aneuploid cells in the tumor. The performed analysis did not reveal any significant differences in the content of aneuploid cells in the tumor (51.9±4.7% and 51.4±5.3%, respectively). Aneuploidy is associated not only with the changes in DNA content in cells but also with the changes in the genome. It is characterized by the DNA index, which is the ratio of the intensity of the fluorescence peak of aneuploid to diploid cells. The results of the analysis of the indices in the groups showed that there were no tumors with the DNA index < 1.0; the DNA index varied from 1.1 to 1.9.
The mean value in the control group was 1.5±0.08, and in the main group, it was – 1.2±0.07. Besides, in the main group, tumors with the DNA index > 1.5 were identified only in 16.7% of cases (p<0.05).
Table 1
Distribution of cells by the phases of the cellular cycle (%)
|
Groups |
G0/G1-phase |
G2+M |
S-phase |
|
Main group |
86.2±3.7 |
2.7±0.6 |
11.2±3.2 |
|
Control group |
87.96±2.18 |
2.12±0.4 |
9.98±1.6 |
Table 2
The ratio of cells by the phases of the cellular cycle of diploid and aneuploid colon tumors (%)
|
Tumor type |
G0/G1-phase |
G2+M-phase |
S-phase |
Proliferation index |
|
Diploid |
86.3±4.3 |
0.11±0.008 v* |
13.7±4.3 |
14.4±3.4 |
|
Aneuploid |
86.2±5 |
3.5±0.9 |
10.4±2.1 |
13.9±3.4 |
Note: * – differences in the parameters were significant concerning aneuploid tumors (p≤0.05).
Table 1 shows the distribution of tumor cells depending on the phase of the cellular cycle. It was established that in the main group, a significant share of tumor cells was at the phase of G0/1 of the cellular cycle (86.2±3.7%) and the phase G2+M – 2.7±0.6%. The rate of cell proliferation was 11.2±3.2%, while the proliferative activity was 13.5±3.2%. In the control group, a similar distribution was observed: most of the cells were at the phase G0/1 – 87.96±2.18%, at the S-phase – 9.98±1.6%, and at G2+M phase – 2.12±0.4%.
The analysis of proliferative activity revealed similar index values in both groups of patients: 13.5±3.2% and 12.1±1.4%, respectively. The authors also analyzed the ratio between diploid and aneuploid tumor cells (Table 2).
Patients with diploid and aneuploid colorectal tumors are characterized by the prevalence of cells at the phase G0/1 of the cellular cycle. The authors revealed significant differences in diploid and aneuploid tumors (p≤0.05): the share of cells in the M and G2 phases was 32 times lower in diploid tumors.
The rate of proliferation in diploid cells exceeded the same parameter in aneuploid cells.
The indices of the proliferation of diploid and aneuploid tumors were similar.
Figs. 1–4 show histograms of the ratio of tumor cells in the studied groups.
Taking into account the fact that the main aim of preoperational radiotherapy in patients with colorectal cancer is a decrease in the primary tumor, the authors also evaluated the clinical effectiveness of the performed radiotherapy (Table 3).
It was revealed that in the group of patients that underwent prolonged radiotherapy, the tumor reduced from 6.8±0.6 cm to 4.5±0.5 cm (p<0.05), and the distance from the anus to the lower border of the tumor increased from 6.3±0.6 cm to 7.6±0.6 cm (p<0.05).
The authors revealed a clinical-morphological effect of pre-operational prolonged radiotherapy in patients with colorectal cancer. Thus, the clinical effectiveness of the therapy manifested itself as a decrease in the colorectal tumor length by 1.5 times and an increase in the distance between the anus and the lower border of the tumor by 1.2 times (p<0.05). Morphologically, these data were confirmed by the development of therapeutic pathomorphism of grade III in 70% of patients. The study at the molecular level revealed significant changes in the content of DNA in tumor cells of the rectum after radiotherapy. A prevalence of tumors with the DNA index < 1.5 by 1.5 times and a decrease in tumors with the DNA index >1.5 by 2.6 times in the main group of patients were observed. Besides, there was a significant decrease in the DNA index from 1.5±0.08 in the control group to 1.2±0.07 in the main group (p≤0.05).
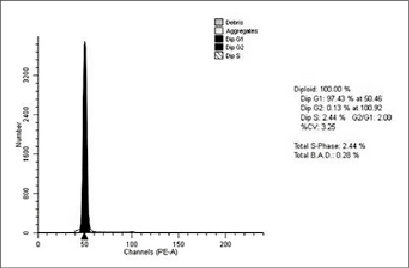
Fig. 1. Histogram of the distribution of cells by the phases of the cellular cycle of a diploid colorectal tumor in the main group. Patient M., female, 62 years old, histologically – G2 adenocarcinoma
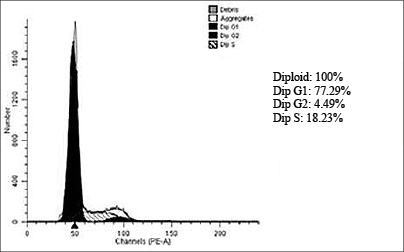
Fig. 2. Histogram of the distribution of cells by the phases of the cellular cycle of a diploid rectal tumor in the control group. Patient M., male, 61 years old, histologically – G2 adenocarcinoma
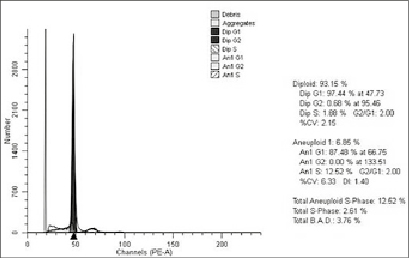
Fig. 3. Histogram of the distribution of cells of an aneuploid rectal tumor in the main group by the phases of the cellular cycle. Patient K., male, 55 years old, histologically – G2 adenocarcinoma
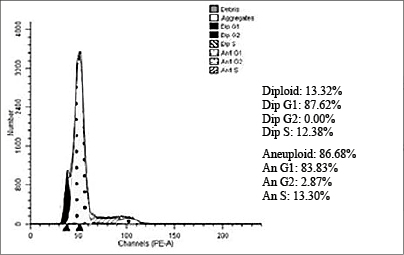
Fig. 4. Histogram of the distribution of cells of an aneuploid rectal tumor in the control group by the phases of the cellular cycle. Patient Sh., female, 47 years old, histologically – G2 adenocarcinoma
Table 3
Characteristics of rectal tumor after a course of radiotherapy
|
Characteristics of the tumor |
Main group |
Control group |
|
Tumor length: before after |
6.8±0.6 cm 4.5±0.5 cm* |
6.0±0.9 cm |
|
Distance from the anus to the tumor: before after |
6.3±0.6 cm 7.6±0.6 cm 5856* |
7.8±0.8 cm |
Note: * – differences in the group are significant (p<0.05).
There were no significant changes revealed in the share of cells at different stages of the cellular cycle in tumors from the main and control groups. Still, radiotherapy led to significant differences between diploid and aneuploid tumors by the share of cells at the stage of mitosis (M) and pre-mitosis (G2) of the cellular cycle that was 32 times lower in diploid than aneuploid tumors (p≤0.05). Pre-operational radiotherapy in patients with colorectal cancer did not reveal significant differences in the share of aneuploid tumors and the mean content of aneuploid cells in the tumor in comparison with the control group. Aneuploid tumors were characterized by the lack of tumors with the DNA index < 1.0 in the main and control groups. The obtained data indicate that 6–8 weeks after prolonged radiotherapy, an expressed clinical morphological effect is observed and the changes at the molecular level at this period correspond to the parameters of a non-irradiated tumor. Thus, an excessive period between the end of the radiotherapy course and surgical intervention can lead to a recurrence of the tumor growth and ineffective neoadjuvant therapy. This fact should be accounted for when planning combined therapy in patients with colorectal cancer.
CONCLUSIONS
1. A decrease in the potential malignancy of colorectal cancer became the main molecular-morphological effect of preoperational radiotherapy. It was observed in the therapeutic pathomorphism of grade III (70%) and an increase in the rate of tumors with the DNA index up to 1.5 by 1.5 times.
2. The clinical effectiveness of preoperational radiotherapy is expressed as a decrease in the length of a colorectal tumor by 1.5 times and an increase in the distance from the anus to the lower border of the tumor by 1.2 times (p<0.05).
FINANCIAL SUPPORT AND SPONSORSHIP Nil.
CONFLICTS OF INTEREST The authors declare no conflict of interest
Библиографическая ссылка
Novikova I.A., Dzhenkova E.A., Shaposhnikov A.V., Gusareva M.A., Kharagezov D.A., Duritskiy M.N., Dashkov A.V., Kolesnikov V.E., Snezhko A.V., Kaymakchi D.O., Mirzoyan E.A., Poluektov S.I., Dontsov V.A., Stateshny O.N. MOLECULAR AND MORPHOLOGICAL EFFECTS OF PROLONGED RADIOTHERAPY IN RECTAL CANCER // Современные проблемы науки и образования. Хирургия. 2020. № 3. С. 9-15;URL: https://clinical-medicine.ru/ru/article/view?id=52 (дата обращения: 04.04.2025).



