Aims
The objective of the study was to investigate the characteristics of diffusion-weighted images (DWI) and pathomorphological features of brain tumours using neuroepithelial and meningeal tumours as examples.
Materials and Methods
The study included data from 76 patients with brain tumour who underwent surgical treatment. All patients underwent brain MRI during the pre-operative phase. Standard T1-, T2-weighted images in three views and DWI in axial view were acquired using 1.5 T Siemens Magnetom Essenza scanner with a b factor of 800 s/mm2 followed by a hardware-based apparent diffusion coefficient (ADC) mapping, which was used to calculate the tumour ADC values using the ROI tool. Cysts and necrosis located in the tumour structure were not included in the measurement area. ADC was calculated in the RadiAnt DICOM Viewer application. Histological and immunohistochemical studies were carried out.
Results
The relationship between the obtained ADC values in the tumour, its cellular density and proliferative potential was identified. The proliferative activity index of tumours (Ki-67) has been shown to be significantly inversely correlated with the ADC values. No significant dependency of the ADC from tumour cell density was discovered in this study. The study showed that at low ADC values, the neuroepithelial tumour has a malignant nature and high proliferative potential, which is characteristic of high-grade tumours (Grade III, IV), as well as that low values of the ADC were characteristic of the anaplastic type of meningiomas (Grade III).
Conclusions
The findings are suggested to be used at the diagnostic stage for differential diagnosis of the nature of a mass discovered in the brain, prediction of its malignancy grade and proliferative potential.
- Fonin V.V., Gribacheva I.A., Dergilev A.P. et al. Differential diagnosis of the multiple focal lesions and expansive process of brain in early stage of the disease [Differentsial’naia diagnostika mnozhestvennogo ochagovogo porazheniia belogo veshchestva i ob”emnogo protsessa golovnogo mozga na rannikh stadiiakh zabolevaniia]. Bulletin of Siberian Medicine - Biulleten’ sibirskoi meditsiny, 2010, vol. 9, no. 4, pp. 165-169, doi: 10.20538/1682-0363-2010-4-165-169.
- Kornienko V.N., Pronin I.N. et al. Diagnostic neuroradiology. Vol. 2 [Diagnosticheskaia neiroradiologiia. Tom 2]. Moscow, Meditsina, 2009, 463 p.
- Chen S.D., Hou P.F., Lou L. et al. The correlation between MR diffusion-weighted imaging and pathological grades on glioma. European Review for Medical and Pharmacological Sciences, 2014. No. 18. pp. 1904–1909.
- Hu Y.C., Yan L.F., Sun Q., et al. Comparison between ultra-high and conventional mono b- value DWI for preoperative glioma grading. Oncotarget, 2017, vol. 8, no. 23, pp. 37884-37895, doi: 10.18632/oncotarget.14180.
- Young R.J., Gupta A., Shah A.D, et al. Potential Role of Preoperative Conventional MRI Including Diffusion Measurements in Assessing Epidermal Growth Factor Receptor Gene Amplification Status in Patients with Glioblastoma. American Journal of Neuroradiology, 2013, vol. 34, no. 12, pp. 2271–2277, doi 10.3174/ajnr.a3604.
- Pronin I.N., Kornienko V.N., Fadeeva L.M. et al. Diffuse-weighted images in the study of brain tumors and peritumoral edema [Diffuzno-vzveshennye izobrazheniia v issledovanii opukholei golovnogo mozga i peritumoral’nogo oteka]. Burdenko’s Journal of Neurosurgery - Voprosy neirokhirurgii imeni N.N.Burdenko, 2002, no. 1, pp. 7-11.
- Serkov S.V. Pronin I.N. Fadeeva L.M. et al. Diffusion-weighted MRI in the diagnosis of neoplasms of the posterior cranial fossa [Diffuzionno-vzveshennaia MRT v diagnostike novoobrazovanii zadnei cherepnoi iamki]. Medical Visualization - Meditsinskaia vizualizatsiia, 2004, no. 2, pp. 66-75.
- Byvaltsev V.A., Stepanov I.A., Kichigin A.I. The Value of Diffusion-weighted Magnetic Resonance Imaging in the Preoperative Evaluation of the Grade of Brain Gliomas. Journal of radiology and nuclear medicine, 2019, vol. 100, no.2, pp. 102-110. DOI: 10.20862/0042-4676-2019-100-2-102-110.
- Byvaltsev V.A., Stepanov I.A., Kichigin A.I. et al. The role of diffusion-weighted mri in differential diagnosis and prediction of survival in patients with brain metastases. Annals of the Russian academy of medical sciences, 2017, vol. 72, no. 6, pp. 442–449, doi 10.15690/vramn890.
- Ginat D.T, Mangla R., Yeaney G. et al. Correlation of diffusion and perfusion MRI with Ki-67 in high-grade meningiomas. American Journal of Roentgenology, 2010, vol. 195, no. 6, pp. 1391–1395, doi: 10.2214/ajr.10.4531.
- Tang Y., Dundamadappa S.K., Thangasamy S. et al. Correlation of apparent diffusion coefficient with Ki67 proliferation index in grading meningioma. American Journal of Roentgenology, 2014, vol. 202, no. 6, pp. 1303–1308, doi 10.2214/AJR.13.11637.
- Gihr G.A., Horvath-Rizea D., Garnov N. et al. Diffusion Profiling via a Histogram Approach Distinguishes Low-grade From High-grade Meningiomas, Can Reflect the Respective Proliferative Potential and Progesterone Receptor Status. Molecular Imaging and Biology, 2018, vol. 20, no. 4, pp. 632-640, doi 10.1007/s11307-018-1166-2.
- Lin L., Bhawana R., Xue Y., et al. Comparative Analysis of Diffusional Kurtosis Imaging, Diffusion Tensor Imaging, and Diffusion- Weighted Imaging in Grading and Assessing Cellular Proliferation of Meningiomas. American Journal of Neuroradiology, 2018, vol. 39, no. 6, pp. 1032-1038, doi 10.3174/ajnr.A5662.
- Sanverdi S.E., Ozgen B., Oguz K.K. et al. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? European Journal of Radiology, 2012, vol. 81, no. 9, pp. 2389–2395, doi 10.1016/j.ejrad.2011.06.031.
INTRODUCTION
Despite modern technical achievements in neurosurgery and radiology, certain difficulties remain in the differentiation of tumors of the brain with similar signaling characteristics [1]. Besides, it is often difficult to estimate visually the borders of the tumor spread at the pre-operative stage to plan the volume of resection and to improve long-term treatment outcomes and decrease the risks of tumor recurrence.
The promising approach to the visualization of tumor infiltration is the method of diffusion-weighted magnetic resonance imaging (DWI) [2]. This method can be used for the differentiation of the area of fast and slow movement of protons. The areas of free and fast protons movement have a higher apparent diffusion coefficient (ADC) [2]. For clinical applications, the areas of structural alterations are relevant that have decreased diffusion in comparison with the surrounding areas, which can be associated with the proliferation of cells and their high density that is typical for neoplastic processes [3–5]. In some cases, the calculation of the ADC allows the specialists to identify the character of the tumor, the areas of its active growth, the borders with healthy brain tissues, and the area of peritumoral edema. Thus, in the morphological study, the areas of the tumor node with high proliferative activity are characterized by higher cellular density and limited diffusion, which is explained by a low volume of the nuclear component in a cell and a bigger volume of the cytoplasm. This is a prognostic factor for the appearance of proliferating vessels and tumor progression [6; 7]. The degree of malignancy of tumors is defined by the immunohistochemical study based on the index of proliferative activity Ki-67. These data are significant in the prognosis and management of such patients [8; 9]. The values of the index of proliferative activity Ki-67 <5% is typical for benign tumors. As a rule, Ki-67 > 5% is associated with cellular anaplasia. The establishment of associations between the ADC of tumor tissues and the degree of their malignancy, cellular density, and index of proliferative activity is an acute and promising direction in neuroradiology [10; 11]. Complex evaluation of central nervous system tumors allows a radiologist to suggest the character of the neoplasm and neurosurgeon to choose a rational tactic for the disease management before the pathomorphological study. Besides, the changes in ADC after chemo and radiotherapy can serve as predictors of the response to the treatment.
The study was aimed to evaluate the possibility of DWI with ADC maps in the diagnosis of tumors of the brain for the evaluation of their proliferative potential and degree of malignancy.
MATERIALS AND METHODS
The study included 76 patients that underwent surgical treatment at the center of neurosurgery in Road clinical hospital (Irkutsk, Russia).
In the diagnostic complex, at the pre-operative stage, all patients had magnetic resonance imaging (MRI) of the brain. Standard T1, T2 weighted images in three projections, and DWI in the axial projection were obtained by 1.5 T tomograph Siemens Magnetom Essenza (Germany) and b-factor 800 c/mm2 with the further building of ADC maps with calculated values of ADC in the tumor by the ROI tool. The area of measurement did not include cysts and necrosis in the structure of the tumor. The ADC was calculated in the RadiAnt DICOM Viewer software.
Histological and immunohistochemical studies were performed in the post-operative period by an experienced pathologist. The cellular density of the tumor was estimated in cells/mm3 in Image J at x400 magnification. For the measurement of the index of proliferative activity (Ki-67), monoclonal antibodies MIB-1 were used (Dako Cytomation, Danmark).
Retrospectively, patients were divided into subgroups according to histological characteristics of tumors by the criteria of classification of the WHO (2016). The study included recurrence-free cases of patients with tumors of the brain: gliomas (39 patients) and meningiomas (37 patients). Gliomas were subdivided by the following grades: GI – no nuclear atypism, mitosis, endothelial proliferation of vessels, necrosis in the structure of the tumor; GII – atypical nuclei or single mitoses; GIII – numerous mitotic figures; GIV – expressed endothelial proliferation of vessels, necroses. Meningiomas were subdivided by the following grades: GI – benign, with a uniform cell pattern and moderate nuclear polymorphism, without foci of necrosis and mitotic activity; GII – atypical, with manifested foci of necrosis and mitotic figures, cells and nuclei polymorphism; GIII – anaplastic with a high density of cells, numerous figures of mitoses and foci of necrosis.
Two groups were formed for each tumor (low ADC and high ADC) with further verification of their uniformity by several criteria (localization, degree of resection, age).
The study protocol followed guidelines for experimental investigation with human subjects in accordance with the Declaration of Helsinki and was approved by the ethics committee. Written informed consent was obtained from each patient (or official representative) before the study.
The analysis of the obtained data was performed in Microsoft Excel 2010 using the methods of descriptive statistics (absolute and relative). The correlation of ADC values, cellular density, and Ki-67 was estimated with Spearman’s significance threshold p = 0.05.
RESULTS
In low malignant gliomas, mean ADC values were 1260 mm2/s. Highly malignant gliomas were characterized by mean low values of ADC – 864 mm2/s.
The comparison of the mean values of the indices of proliferative activity Ki-67 of low and highly malignant tumors revealed statistically significant differences. In low malignant gliomas, Ki-67 varied from 2 to 12% (on average, 5%); in gliomas, Ki-67 was high and varied from 12 to 97% (on average, 58%).
Benign meningiomas had a high ADC (on average, 1375 mm2/s). Atypical meningiomas had intermediate values of ADC (on average, 1113 mm2/s). Anaplastic meningiomas were characterized by a low ADC (on average, 689 mm2/s). The analysis of ADC values did not reveal any significant difference in the intensity of the signal in the structure of benign and anaplastic meningiomas. Still, the intensity of the signal on ADC maps of benign meningiomas significantly differed from anaplastic meningiomas. The values of the index of proliferative activity of Ki-67 in meningeal tumors varied from 1% to 17% (on average, 4%). The analysis of the data showed statistically significant differences between all the groups with meningeal tumors.
The authors obtained data on the reverse correlation between the ADC and indices of proliferative activity of Ki-67 in gliomas and meningiomas (Fig. 1).
The calculation of cellular density in gliomas showed variable values without significant differences (low-grade malignancy from 594 to 1785 cells/mm3, high-grade malignancy from 657 to 1783 cells/mm3).
The density of cells in meningiomas (from 457 to 1785 cells/mm3) also varied widely in each group and did not show a significant correlation with the degree of their malignancy.
The obtained ADC values and cellular density in gliomas and meningiomas are presented in Fig. 2.
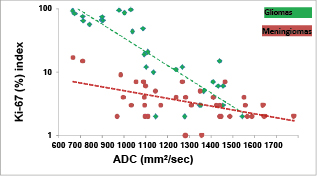
Fig. 1. Correlation of the ADC and Ki-67 in gliomas and meningiomas of different degrees of malignancy
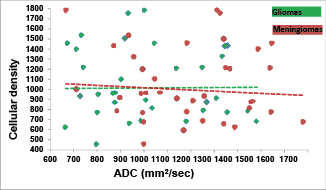
Fig. 2. ADC and cellular density in gliomas and meningiomas
The study results showed that in high-grade malignant gliomas, ADC values were significantly lower than in low-grade malignant gliomas. Thus, there is a statistically significant correlation between the ADC and Ki-67 in neuroepithelial tumors. This indicates the fact that ADC reflects a pathomorphological picture of a glioma.
The obtained data agreed with other authors. Thus, Chen et al. showed different ADC values in low-grade and high-grade malignant gliomas with signs of reserve correlation [3]. The same results were obtained by Hu et al. [4]. In the study by Serkov et al. [7], the mean values of the ADC for low-grade malignant astrocytomas were 1520 mm2/s, which was associated with low cellular density. The mean values of ADC for anaplastic astrocytomas were 1180–1230 mm2/s [7]. However, these studies stated that the ADC depended on cellular density, which was not confirmed by the present study. In the present study, high-grade malignant gliomas had ADC < 1340 mm2/s. The consolidation of the earlier obtained and newly revealed data allowed the authors to suggest that low ADC values were typical for malignant gliomas with high proliferative potential.
The present study demonstrated that in meningiomas, the ADC and Ki-67 had a statistically significant reverse correlation. Thus, it can be concluded that the ADC reflects a pathomorphological picture of a meningioma.
The obtained data agreed with the results of other studies that also showed a correlation between the ADC and proliferative potential of meningiomas [10–13]. In the study by Tang et al. [11], the threshold value of the ADC in the differential diagnosis of meningiomas GI, GII, and GIII was 850 mm2/s. In the present study, anaplastic meningiomas had ADC < 711 mm2/s. Thus, this range of values allowed the authors to suggest the anaplastic nature of meningioma. However, not all researchers obtained similar data. Some studies did not reveal significant differences between the ADC and meningiomas of different types [14].
The differences in the obtained data can be explained by different methods of the study. Despite some differences in the obtained data, the results of the present study primarily agreed with other researchers. The present study had some limitations because of retrospective design and small representing sampling. The subject requires a further study on a larger sampling with a complex analysis of the data for tumors of the brain of all the histological types.
CONCLUSIONS
The performed study clearly showed that the intensity of the signal on ADC maps and the parameters of the proliferative potential of the tumor (Ki-67) for neuroepithelial tumors of low and high grade of malignancy and for meningiomas had significant differences. The tumors with high proliferative potential were characterized by a low intensity of a signal on an ADC map, the tumor with low proliferation potential – a high-intensity signal on an ADC map. Thus, MRI with DWI and ADC mapping in a complex diagnosis of tumors of the brain allows the specialists to predict the character of the revealed disease at the diagnostic stage, assume the degree of its malignancy and proliferative potential, and predict the possibility of tumor recurrence. Timely application of all the possibilities of modern neuro-oncology allows for the best possible degree of tumor resection and, thus, elongation of the recurrence-free period and an increase in the general survival rate in this category of patients.
CONFLICTS OF INTEREST The authors declare no conflict of interest
SUPPLEMENTARY DATA (DOI)
Библиографическая ссылка
Byvaltsev V.A., Dergilev A.P., Kichigin A.I., Kanygin V.V. DIFFUSION-WEIGHTED MAGNETIC RESONANCE IMAGING IN DIFFERENTIAL DIAGNOSIS OF BENIGN AND MALIGNANT BRAIN TUMOURS // Современные проблемы науки и образования. Хирургия. 2020. № 2. С. 19-23;URL: https://clinical-medicine.ru/ru/article/view?id=48 (дата обращения: 04.04.2025).



