Aims
The aim of this study was evaluating the correlation between the degree of expression of protein kinase M in the tumour samples of patients with pilocytic astrocytoma (grade I) and the parameters of non-relapse survival rate.
Materials and Methods
The effect of the degree of expression of brain-specific atypical isoforms of protein kinase C - protein kinase M (PK M) on the prognosis of non-relapse survival rate for patients diagnosed with pilocytic astrocytoma has been studied. Two equal groups: one with at least 5-year relapse-free period (RFG [relapse free group]) and one with the relapse (RG [relapse group]) have been formed. Rabbit monoclonal antibodies against human PK M antigen have been used for analysis.
Results
It has been shown that the percentage of cells expressing PK M (PKE M) was statistically significantly higher in the RG, unlike in the RFG (34.36±1.52% and 24.14±1.25% accordingly). Also, the value of the histoscore of PK M was significantly higher in the RG as compared to the RFG, 120.46±3.6% and 91.7±3.31%. Moreover, a high-level reverse correlation between the parameters of PKE M and the histoscore of PK M and time prior to relapse has been found.
Conclusions
These results show the presence of the correlation between the degree of PK M expression and progress of the tumour process, and can also become the basis for creation of new diagnostic approaches and development of means of target therapy of pilocytic astrocytoma.
- Jones T., Hutter B., Jäger N. et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature. Genetics, 2013, vol. 45, no. 8, pp. 927-932, doi: 10.1038/ng.2682.
- Louis D.N., Perry A., Reifenberger G. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica. 2016, vol. 131, no. 6, pp. 803-820, doi 10.1007/s00401-016-1545-1.
- Matyja E., Grajkowska W., Stępień K. et al. Heterogeneity of histopathological presentation of pilocytic astrocytoma - diagnostic pitfalls. A review. Folia Neuropathologica. 2016, vol. 54, no 3, pp. 197-211, doi 5114/fn.2016.62530.
- Zeng L., Webster S.V., Newton P.M. The biology of protein kinase C. Advances in Experimental Medicine and Biology. 2012, vol. 740, pp. 639-661, doi 10.1007/978-94-007-2888-2_28.
- Hernandez A.I., Blace N., Crary J.F. et al. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. Journal of Biological Chemistry, 2003, vol. 278, no 41, pp. 40305-40316, doi 10.1074/jbc.m307065200.
- Sacktor T.C. 2 PKMζ, LTP maintenance, and the dynamic molecular biology of memory storage. Progress in Brain Research, 2008, vol. 169, pp. 27-40. doi 10.1016/s0079-6123(07)00002-7.
- Wang Y.X., Zhang X.R., Zhang Z.J. et al. Protein kinase Mζ is involved in the modulatory effect of fluoxetine on hippocampal neurogenesis in vitro. The International Journal of Neuropsychopharmacology, 2014, vol. 17, no. 9, pp. 1429-1441, doi 10.1017/S1461145714000364.
- Hartsink-Segers S.A., Beaudoin J.J., Luijendijk M.W. et al. PKCζ and PKMζ are overexpressed in TCF3-rearranged paediatric acute lymphoblastic leukaemia and are associated with increased thiopurine sensitivity. Leukemia, 2015, vol. 29, no. 2, pp. 304-311, doi 10.1038/leu.2014.210.
INTRODUCTION.
The term “pilocytic” has been used since 1930 for the description of astrocytomas that contain bipolar cells with piliform processes [1]. What is now called pilocytic astrocytoma (PA) used to have numerous names before the WHO system of classification became generally accepted. Before, PA was called polar spongioblastoma and juvenile astrocytoma. PA is a tumor of low malignancy (Grade I by the WHO classification of tumors of the central nervous system (CNS)).
Pilocytic astrocytoma is the most common brain tumor in children and adolescents (33.2% of the total number of gliomas that develop in patients aged 0-14 years old and 17.6% from the total number of primary brain tumors in children) [2]. The mean age of the onset in the senior age group is 22 years old. Patients older than 50 years have PA much rarer.
PA develops in different parts of the CNS affecting the optic nerve, optic chiasma/ hypothalamus, thalamus and basal ganglia, brain hemispheres, and brainstem. However, in the majority of cases, PA is localized subtentorially.
PA is characterized by a bi-phase pattern that includes closely aligning bipolar cells that contain aggregates of α-B-crystallin in the form of Rosenthal fiber and loose multipolar cells with microcysts and eosinophilic grains that contain α1-antichymotrypsin and α1-antitrypsin. These two elements can be observed in different proportions providing a significant histological diversity. It should be noted that it is possible to reveal the features characteristic of high-grade neoplasms, for example, cellular and nuclear anaplasia, the proliferation of the vascular endothelium, necrotic foci, etc. However, these features often represent degenerative alterations caused by the slow growth of the tumor and not malignization [3].
Pilocytic astrocytoma has a wide variety of genetic aberrations. The fusion BRAF-KIAA1549 which induces the MAPK cascade is revealed in more than 70% of tumors. There are also other fusions of the gene BRAF, its deletion and mutation of V600E revealed. All these genetic deviations activate the MAPK cascade, which leads to an increase in the proliferative activity of the cells. Other mechanisms of activation of this signal pathway include sporadic inactivating mutation of the gene NF1 or mutation of two alleles of the gene in patients with type-I neurofibromatosis, KRAS mutations, point mutations N546K and K656E of the gene FGFR1 and its fusion with the gene TACC1; mutation of the gene RAF1 including a rare fusion with the gene SRGAP3, and mutations in the genes of the NTRK family [2].
There are a lot of data on the genetic origin of PA. However, secondary mechanisms of progressing of these tumors remain understudied. These mechanisms play an important role in the realization of the proliferative potential of tumor cells due to the mutations that lead to tumor growth and progression. Understanding of these mechanisms is important from the fundamental point of view because it improves the knowledge on the grounds of carcinogenesis. The practical value of this knowledge is explained by the fact that radical resection is not always possible because of complicated localization of the tumor, which often leads to the recurrence of the pathological process. Presently, there are neither reliable tools of prognosis of PA development nor effective target therapy for the treatment of such patients.
At the same time, the factors involved in the process of tumor progression should possess specific properties, for example, the capability of quick activation and long-term maintenance of plastic molecular transformations in the tumor cells. These properties can be observed in a special type of atypical isoform of protein kinase C – protein kinase Mζ (PK Mζ). PK Mζ is an alternative splicing variant of PKC ζ that does not contain a regulatory auto-inhibiting domain. For this reason, it remains active until it gets destructed [4; 5]. At the same time, PK Mζ is specific for the brain tissue [6; 7]. In general, the role of PK Mζ in patients with tumors is hardly studied. It was revealed that in patients with acute lymphocytic leukemia, PK Mζ is synthesized in tumor cells and gets involved in the process of carcinogenesis [8]. However, in patients with brain tumors, the role of this factor has not been studied. Thus, the present study was aimed to evaluate the association between the expression of PK Mζ in PA and the recurrence-free survival.
MATERIALS AND METHODS
Groups and their characteristics
The retrospective, blind, randomized study included 50 samples of tumors obtained from patients diagnosed with pilocytic astrocytoma (PA) (Grade I by the WHO) that underwent surgical treatment at the Burdenko National Medical Research Center of Neurosurgery in 2009–2014. These patients were followed up for minimum 5 years. They underwent regular control MRI of the brain for a possible tumor recurrence (minimum twice a year). There were two equal groups of patients (25 patients in each group): a group of patients with tumor recurrence (RG) and a group of patients without tumor recurrence (NRG) for at least 5 years. The tumor recurrence was diagnosed in patients with a clinical picture of the tumor in the typical localization and evident MR signs of the tumor recurrence in the same place as the primary tumor. The time to the recurrence was calculated as days from the surgery for the primary tumor to the registration of the signs of the recurrence.
The mean age of patients included in the study was 11.55±1.54 years old. There were 52% of men and 48% of women. In the RG, the mean age of patients was 8.95±1.68 years old. In the NRG, it was 14.15±2.51 years old. In the RG, the mean time to the recurrence was 819.92±126.72 days.
The study protocol followed guidelines for experimental investigation with human subjects in accordance with the Declaration of Helsinki and was approved by the ethics committee. Written informed consent was obtained from each patient (or an official representative) before the study.
Immunohistochemical Study
The immunohistochemical study was performed using waxed blocks with fixed samples of the tumor (in the RG, the samples of the tumor were taken after the 1st surgery) that were used for preparing 3-µm-thick sections. The sections were dewaxed with xylene and hydrated with different solutions of ethanol. After that, the obtained preparations were incubated with rabbit monoclonal antibodies to the antigen of human PK Mζ (Abcam, Great Britain) and anti-rabbit murine IgG against horseradish peroxidase (Cell Marque, Sigma-Aldrich, USA). The sites of binding were revealed using diaminobenzidine 3,3'-tetrahydrochloride (Ventana Medical Systems, USA).
Processing and analysis of the immunohistochemical study results
The samples were studied with a light microscope at x400 magnification by three pathologists. For the evaluation of the activity of the expression of PK Mζ, the calculation of the ratio of cells that expressed this marker (PK Mζ) was performed. In addition, the expression was evaluated with a histoscore semiquantitative method. The mean values were recorded.
For the calculation of the histoscore of PK Mζ, the share of weakly stained cells was multiplied by 1, the share of moderately stained cells was multiplied by 2, the share of the cells with expressed positive staining was multiplied by 3, and the results were summed. For the evaluation of the expression of the marker, the cytocolorimetric method was used and a microscope Carl Zeiss Scope.A1, a camera Axiocam 105 color (Zeiss AG, Germany), and the software for the formation and analysis of the images ZEN 2 (Zeiss AG, Germany), ImageJ (NIH, USA) and Adobe Photoshop (Adobe Systems, USA). The mean values of the expression were recorded by three pathologists.
Statistical processing of the study results
Statistical processing of the study results was performed with Statistica 10 (StatSoft, USA). The significance of the differences in the activity of the expression of PK Mζ in two groups was evaluated with the Mann-Whitney U-test. For the estimation of the influence of PK Mζ and histoscore PK Mζ on the survival, correlation analysis between these two parameters was performed by the Spearman test. The differences were significant at p<0.05.
RESULTS AND DISCUSSION
Results
The share of cells with positive expression of PK Mζ in both groups
The analysis of the results showed that the expression of PK Mζ in the NRG was 24.14±1.25%. At the same time, the mean expression of PK Mζ in the RG was higher and equal to 34.36±1.52% (Figure 1). The revealed differences were statistically significant (p=0.000034, z=4.14).
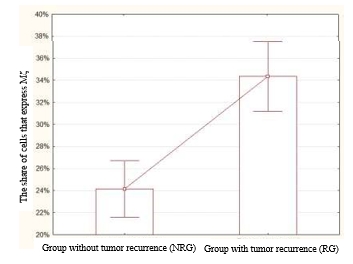
Fig. 1. The share of cells that express PK Mζ in the NRG and RG. The level of expression of this marker was significantly higher in the RG than in the NRG (p=0.000034, z=4.14)
Histoscore PK Mζ in the NRG and RG
The mean histoscore of PK Mζ in the NRG was 91.7±3.31%. At the same time, the mean histoscore PK Mζ in the RG was higher and was equal to 120.46±3.6% (Figure 2). The revealed differences were significantly different (p=0.000006, z=4.51).
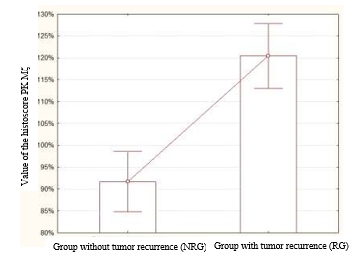
Fig. 2. Values of the histoscore PK Mζ in the NRG and RG. This parameter was significantly higher in the RG than in the NRG (p=0.000006, z=4.51)
Correlation between the expression of PK Mζ and the histoscore PK Mζ and time to recurrence
A negative correlation was revealed between the expression of PK Mζ and the time to recurrence (r=−0.9488) (Figure 3A). A negative correlation was also revealed between the histoscore PK Mζ and time to recurrence (r=−0.9994) (Figure 3B).
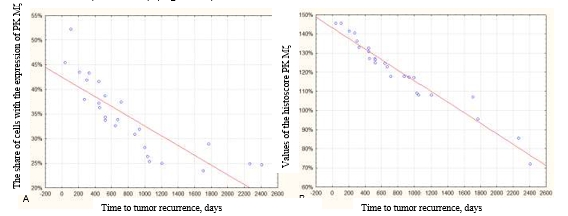
Fig. 3. Correlation between the expression of PK Mζ and time to the tumor recurrence. A – negative correlation between the share of cells positive to the marker PK Mζ and time to tumor recurrence (r=−0.9488). B – negative correlation between the values of the histoscore PK Mζ and time to tumor recurrence (r=−0.9994)
Discussion
Genetic modifications and transformations in patients with PA are actively studied. In cases with PA, the most important role in the transformations is played by the gene BRAF. The fusion KIAA1549-BRAF is the most common. The mutation BRAF V600E is met much rarer.
Still, the most important pathogenic mechanisms that directly influence the tumor progression and determine the functional activity of single tumor cells remain understudied. In this aspect, effector mechanisms that contribute to the realization of the main oncologic potential in the driver mutations and oncogene transformations play a crucial role.
The study results showed that PK Mζ could play an important role in the progression of the tumor process. It was possible to reveal the differences in the expression of PK Mζ between the group of patients with recurrence-free PA in the post-operative period and the group of patients with PA recurrence. Besides, a strong correlation was established between the expression of PK Mζ and the time to tumor recurrence.
CONCLUSIONS
A potentially valuable marker for the prognosis of recurrence-free survival was identified, which may contribute to the development of new diagnostic and follow-up approaches. Fundamentally, it was shown that PK Mζ can play a center effector pathogenetic role in the development of pilocytic astrocytomas because the level of its expression correlates with the progression of the tumor process. The key role of PK Mζ in the carcinogenesis makes it a promising target for the development of target therapy drugs.
FINANCIAL SUPPORT AND SPONSORSHIP
The study was financed by The Russian Foundation for Basic Research within the scientific project No. 18-29-01034 mk.
CONFLICTS OF INTEREST
The authors declare no conflict of interest
Библиографическая ссылка
Nikitin P.V., Ryzhova M.V., Muradyan M.A., Galstyan S.A., Zubova I.V. PILOCYTIC ASTROCYTOMA AND PROTEIN KINASE IS THERE A CORRELATION WITH THE OUTCOME? // Современные проблемы науки и образования. Хирургия. 2020. № 1. С. 19-23;URL: https://clinical-medicine.ru/ru/article/view?id=43 (дата обращения: 04.04.2025).



